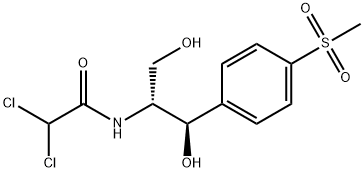
Thiamphenicol
- CAS No.
- 15318-45-3
- Chemical Name:
- Thiamphenicol
- Synonyms
- thiophenicol;D-THREO-2,2-DICHLORO-N-(BETA-HYDROXY-ALPHA-[HYDROXYMETHYL]-4-[METHYLSULFONYL]PHENETHYL)ACETAMIDE;Neomyson;8065c.b.;NSC 522822;win-5063-2;thiocymetin;hiamphenicol;D-Thiocymetin;THIAMPHENICOL
- CBNumber:
- CB0102588
- Molecular Formula:
- C12H15Cl2NO5S
- Molecular Weight:
- 356.22
- MDL Number:
- MFCD00467983
- MOL File:
- 15318-45-3.mol
| Melting point | 163-166 °C |
|---|---|
| alpha | D25 +12.9° (ethanol) |
| Density | 1.3281 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | ethanol: 50 mg/mL, clear, colorless |
| form | powder |
| Boiling point | 695.9±55.0 °C(Predicted) |
| pka | 11.05±0.46(Predicted) |
| color | white to off-white |
| Water Solubility | Soluble in acetonitrile or DMF. Slightly soluble in water |
| Merck | 14,9301 |
| BRN | 2819542 |
| InChIKey | OTVAEFIXJLOWRX-NXEZZACHSA-N |
| CAS DataBase Reference | 15318-45-3 |
| FDA UNII | FLQ7571NPM |
| ATC code | J01BA02,J01BA52 |
| EPA Substance Registry System | Thiamphenicol (15318-45-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | AB6680000 | |||||||||
| HS Code | 29414000 | |||||||||
| Toxicity | human,TDLo,unreported,214mg/kg/10D (214mg/kg),BEHAVIORAL: SLEEPGASTROINTESTINAL: NAUSEA OR VOMITINGSKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE",Arzneimittel-Forschung. Drug Research. Vol. 24, Pg. 944, 1974. | |||||||||
| NFPA 704 |
|
Thiamphenicol price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T0261 | Thiamphenicol | 15318-45-3 | 1g | $70.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | T0261 | Thiamphenicol | 15318-45-3 | 5g | $204 | 2024-03-01 | Buy |
| TCI Chemical | T2802 | Thiamphenicol >98.0%(HPLC)(N) | 15318-45-3 | 5g | $64 | 2024-03-01 | Buy |
| TCI Chemical | T2802 | Thiamphenicol >98.0%(HPLC)(N) | 15318-45-3 | 25g | $189 | 2024-03-01 | Buy |
| Alfa Aesar | J63575 | Thiamphenicol | 15318-45-3 | 1g | $30.6 | 2024-03-01 | Buy |
Thiamphenicol Chemical Properties,Uses,Production
Description
Thiamphenicol is a broad-spectrum antibiotic chloramphenicol, which is more effective to the gram-negative bacteria than the gram-positive bacteria. At room temperature, it is a white to off-white crystalline powder or crystal, which can be quickly and completely absorped by oral adminstration, as well as it is excreted mainly in the prototype from the urine for metabolism. It is clinically applied for the treatment of respiratory, urinary tract, liver and gallbladder, typhoid and other intestinal surgery, gynecology and ENT infections. Especially in the mild infections it is more effective. It has the similar chemical structure with the chloramphenicol. Its methyl sulfone substituted the nitro of chloramphenicol, which reduced its toxicity, and in vivo its antibacterial activity is 2.5-5 times stronger than chloramphenicol. For gram-positive bacteria, such as streptococcus pneumoniae and hemolytic streptococcus, it has very strong antibacterial effect, while for gram-negative bacteria, such as Neisseria gonorrhoeae, meningococcus, lung Bacteroides, E. coli, Vibrio cholerae, Shigella and influenza bacillus, it also has strong antibacterial effect. For anaerobic bacteria, Rickettsia and amoeba, it has antibacterial effect in some extent. It has the same antimicrobial mechanism with chloramphenicol, which mainly inhibits the synthesis of bacterial protein. This drug is absorped quickly by oral administration, which reaches peak blood concentration within two hours. Its half-life is 5 hours, that is more longer than chloramphenicol. The bacteria have complete cross resistance to it and chloramphenicol, while the bacteria have some cross-resistance phenomenon to it and tetracycline.
Thiamphenicol also has strong immunosuppressive effects, which is an excellent immunosuppressant. Its mechanism of action have significantly different with other immunosuppressive agents. The immunosuppressive effect is several times higher than the chloramphenicol. It can be as the effective extender for transplantation reaction and surgically allogeneic transplantation.
Chemical Properties
White to off-white crystalline powder or crystal. Melting point (℃) 178-180 (swirled), 164-166 (right-handed).
Uses
It is applied for the treatment of respiratory, urinary tract, liver and gallbladder, typhoid and other intestinal surgery, gynecology and ENT infections. Especially in the mild infections it is more effective.
Chemical Properties
Off-White Solid
Originator
Thiophenicol,Clin Midy,France,1967
Uses
chelating agent, antiseborrheic
Uses
Antimicrobial
Uses
Thiamphenicol is a semi-synthetic chloramphenicol prepared by total synthesis from thiophenol in which the nitro moiety of chloramphenicol is replaced by a methylsulphone, first synthesised at Sterling Winthrop in 1952. Thiamphenicol is a broad spectrum antibiotic with good activity against Gram negative and anaerobic bacteria. Thiamphenicol acts by binding to the 23S sub-unit of the 50S ribosome inhibiting protein synthesis. Thiamphenicol has been extensively studied with over 800 literature citations.
Uses
Thiamphenicol is an antibiotic. Thiamphenicol is the methyl-sulfonyl analogue of chloramphenicol and has a similar spectrum of activity, but is 2.5 to 5 times as potent. Thiamphenicol is used particul arly for the treatment of sexually transmitted infections and pelvic inflammatory disease.
Definition
ChEBI: Thiamphenicol is a sulfone and a monocarboxylic acid amide. It has a role as an immunosuppressive agent and an antimicrobial agent.
Manufacturing Process
A mixture of 50 parts by weight of racemic 2-acetylamino-1-(4-
methylmercaptophenyl)-1,3-propanediol, 100 parts by weight of concentrated
hydrochloric acid, and 500 parts by weight of water was warmed on a steam
bath for thirty minutes. The resulting solution was cooled to about 40°C and
was then made strongly alkaline by addition of 35% aqueous sodium
hydroxide solution. The alkaline solution was then refrigerated. The white solid
which separated from the cooled solution was collected on a filter. There was
thus obtained 27 parts by weight of 2-amino-1-(4-methylmercaptophenyl)-
1,3-propanediol. This product melted at 130.7°C to 131.9°C after
recrystallization from methanol.
This compound was converted to the tartrate and the optical isomers were
resolved.
A mixture of 1.1 g of 2-amino-1-(4-methylmercaptophenyl)-1,3-propanediol,
obtained as described above and 1.6 ml of ethyl dichloroacetate was heated
on a steam bath for three hours. The resulting viscous yellow oil was
dissolved in 25 ml of ethylene chloride and filtered hot with charcoal, and the
filtrate was allowed to cool to about 25°C. From the filtrate there separated
0.92 g of tiny white leaflets which were collected on a filter. Recrystallization
of this product, which was a dextro-rotary form of 2-dichloroacetylamino-1-(4-
methylmercaptophenyl)-1,3-propanediol from nitroethane yielded the pure
product, which melted at 111.6°C to 112.6°C.
7 g of the 2-dichloroacetylamino-1-(4-methylmercaptophenyl)-1,3-propanediol
obtained as described above was dissolved in 30 ml of acetone. To this
solution there was added dropwise with stirring 10 ml of 40% peracetic acid.
The temperature during the reaction was maintained at 39°C to 45°C by
cooling the reaction vessel. After stirring the mixture for two hours, it was
diluted with 100 ml of water and the solution allowed to stand over the
weekend in the refrigerator. The solid which separated from solution was
collected on a filter, washed several times with ice water, and dried overnight
at 70°C.
Therapeutic Function
Antibacterial
Antimicrobial activity
It is generally less active than chloramphenicol, but is equally active against Str. pyogenes, Str. pneumoniae, H. influenzae and N. meningitidis, including some strains resistant to chloramphenicol. It is more actively bactericidal against Haemophilus and Neisseria spp.
Acquired resistance
There is complete cross-resistance with chloramphenicol in those bacteria which elaborate acetyltransferase, although the affinity of the enzyme for thiamphenicol is lower. Organisms that owe their resistance to other mechanisms may be susceptible.
Pharmacokinetics
An oral dose of 500 mg produces a peak plasma level of
3–6 mg/L after about 2 h. The plasma half-life is 2.6–3.5
h. It is said to reach the bronchial lumen in concentrations
sufficient to exert a bactericidal effect on H. influenzae.
Unlike chloramphenicol it is not a substrate for
hepatic glucuronyl transferase; it is not eliminated by conjugation,
and its half-life is not affected by phenobarbital
induction.
About 50% of the dose can be recovered in an active form
in the urine within 8 h and 70% over 24 h. The drug is correspondingly
retained in the presence of renal failure, and
in anuric patients the plasma half-life has been reported to
be 9 h, a value not significantly affected by peritoneal dialysis.
Biliary excretion is believed to account for removal of
the antibiotic in anuric patients. The plasma concentration
is elevated and half-life prolonged in patients with hepatitis
or cirrhosis.
Clinical Use
Similar to that of chloramphenicol.
Side effects
There are no reports of irreversible bone-marrow toxicity. This has been related to the absence of the nitro group, and hence its reduction products, and differences in the biochemical effects of thiamphenicol and chloramphenicol on mammalian cells. It exerts a greater dose-dependent reversible depression of hemopoiesis and immunogenesis than chloramphenicol, and has been used for its immunosuppressive effect. Therapeutic doses (1–1.5 g) are likely to depress erythropoiesis in the elderly or others with impaired renal function.
Purification Methods
Recrystallise thiamphenicol from H2O or CHCl3. The UV has max at 224, 266 and 274nm ( 13,700, 800 and 700) in 95% EtOH. The 1S,2S-isomer [1478651-7] has m 164.3-166.3o (from H2O/EtOAc/pet ether) and [] D 25 -12.6o (c 1, EtOH); and the racemate 1RS,2RS-Racefenical [847-25-6] has m 181-183o (dec) from CHCl3/EtOAc/pet ether. [Cutler et al. J Am Chem Soc 74 5475, 5482 1952, UV: Nachod & Cutler J Am Chem Soc 74 1291 1952, Suter et al. J Am Chem Soc 75 4330 1953, Cutler et al. J Am Pharm Assoc 43 687 1954, Beilstein 13 IV 2957.]
Thiamphenicol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3958 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8053 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +86-18239973690 +86-18239973690 | sales@suikangpharm.com | China | 311 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
View Lastest Price from Thiamphenicol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Thiamphenicol
15318-45-3
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min | 500kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-22 | Thiamphenicol
15318-45-3
|
US $0.00 / kg | 25kg | 99% | 10tons | Henan Suikang Pharmaceutical Co.,Ltd. | |
 |
2024-11-19 | Thiamphenicol
15318-45-3
|
US $0.00 / g | 1g | 98% HPLC | 100kg | shandong perfect biotechnology co.ltd |
-

- Thiamphenicol
15318-45-3
- US $0.00-0.00 / Kg/Drum
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Thiamphenicol
15318-45-3
- US $0.00 / kg
- 99%
- Henan Suikang Pharmaceutical Co.,Ltd.
-

- Thiamphenicol
15318-45-3
- US $0.00 / g
- 98% HPLC
- shandong perfect biotechnology co.ltd
15318-45-3(Thiamphenicol)Related Search:
1of4