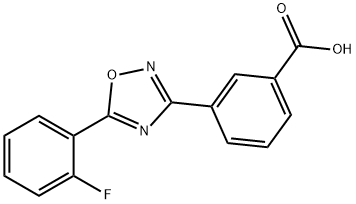
Ataluren
- CAS No.
- 775304-57-9
- Chemical Name:
- Ataluren
- Synonyms
- Ataluren;Ptc124;CS-749;Ptc-124;Ptc 124;Atarulon;Ataluren [usan];Ataluren, >=98%;PTC-124,ataluren;Ataluren (PTC124)
- CBNumber:
- CB02128667
- Molecular Formula:
- C15H9FN2O3
- Molecular Weight:
- 284.24
- MDL Number:
- MFCD09864996
- MOL File:
- 775304-57-9.mol
- MSDS File:
- SDS
| Melting point | 241 - 242°C |
|---|---|
| Boiling point | 503.7±60.0 °C(Predicted) |
| Density | 1.379 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly) |
| form | White to off-white solid. |
| pka | 3.58±0.10(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C15H9FN2O3/c16-12-7-2-1-6-11(12)14-17-13(18-21-14)9-4-3-5-10(8-9)15(19)20/h1-8H,(H,19,20) |
| InChIKey | OOUGLTULBSNHNF-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C1=CC=CC(C2N=C(C3=CC=CC=C3F)ON=2)=C1 |
| CAS DataBase Reference | 775304-57-9 |
| FDA UNII | K16AME9I3V |
| ATC code | M09AX03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H336-H373 |
| Precautionary statements | P260-P271-P304+P340+P312-P314-P403+P233-P405 |
| HS Code | 2933998090 |
Ataluren price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 5.30918 | PTC-124 - CAS 775304-57-9 - Calbiochem | 775304-57-9 | 25MG | $219 | 2023-01-07 | Buy |
| Cayman Chemical | 16758 | PTC-124 ≥98% | 775304-57-9 | 5mg | $49 | 2024-03-01 | Buy |
| Cayman Chemical | 16758 | PTC-124 ≥98% | 775304-57-9 | 10mg | $87 | 2024-03-01 | Buy |
| Cayman Chemical | 16758 | PTC-124 ≥98% | 775304-57-9 | 25mg | $190 | 2024-03-01 | Buy |
| Cayman Chemical | 16758 | PTC-124 ≥98% | 775304-57-9 | 50mg | $310 | 2024-03-01 | Buy |
Ataluren Chemical Properties,Uses,Production
Description
Ataluren (PTC124)Cas:775304-57-9 selectively induces ribosomal read-through of premature but not normal termination codons, with EC50 of 0.1 μM in HEK293 cells, may provide treatment for genetic disorders caused by nonsense mutations (e.g. CF caused by CFTR nonsense mutation). Phase 3.
Features
Demonstrates oral bioavailability, and an appropriate safety toxicology profile.
In vitro
Compared with Gentamicin which is only active at much higher concentrations, PTC124 is a more potent nonsense-suppressing agent and exhibits 4-to 15-fold stimulation of read-through relative to controls. PTC124 (0.01-3 μM) promotes dose-dependent read-through of all three nonsense codons in HEK293 cells harboring LUC-190 nonsense alleles with the highest read-through at UGA, followed by UAG and then UAA, but it does not suppress multiple proximal nonsense codons. Like Gentamicin, PTC124 is most active when a pyrimidine (in particular cytosine, C) follows the nonsense codon. Consistent with the stable cell line reporter assay, PTC124 (17 μM) promotes significant production of dystrophin in primary muscle cells from Duchenne muscular dystrophy (DMD) patients or MDXMDX mice expressing dystrophin nonsense alleles. PTC124 selectively promotes ribosomal read-through of premature termination but not normal termination codons, even at concentrations substantially greater than the values achieving maximal activity.
In vivo
Due to functional recovery of dystrophin production, oral, intraperitoneal or combined dosing of PTC124 for 2-8 weeks partially rescues functional strength deficit in dystrophic muscles of MDX mice, and results in partial protection against contraction-induced injury in the extensor digitorum longus (EDL) muscles, as well as significant reductions in serum creatine kinase values. In Cftr-/-mice expressing a human CFTR-G542X transgene, subcutaneous or oral administration of PTC124 (~60 mg/kg) suppresses the G542X nonsense mutation in a dose-dependent manner, leading to a significant restoration of human (h)CFTR protein expression and function without any effect on nonsense-mediated mRNA decay (NMD) or other aspects of mRNA stability. PTC124 treatment (60 mg/kg) restores 29% of the normal intestinal transepithelial cAMP-stimulated shortcircuit currents observed in Cftr+/+ mice, displaying a significant advantage compared with Gentamicin.
Description
Ataluren is a drug marketed under the trade name Translarna® which was developed by PTC Therapeutics and approved by the European Union in May 2014 for the treatment of Duchenne’s muscular dystrophy (DMD) and potentially other genetic disorders. Ataluren renders ribosomes less sensitive to premature stop or ‘read-through’ codons, which are thought to be beneficial in diseases such as DMD and cystic fibrosis.
Description
Nonsense mutations create a premature termination of mRNA translation and have been implicated in various genetic disorders, including muscular dystrophy and cystic fibrosis. PTC-124 is a nonaminoglycoside that has been reported to selectively induce ribosomes to read through premature nonsense stop signals on mRNA, thus allowing the production of full length, functional proteins. In a mouse model of cystic fibrosis caused by nonsense mutations, PTC-124 treatment (60 mg/kg s.c. injection or 0.3-0.9 mg/ml orally) has been shown to restore cystic fibrosis transmembrane conductance regulator (CFTR) protein expression and function. The target activity of PTC-124 was initially evaluated by firefly luciferase reporter cell-based nonsense codon assay (IC50 = 7 nM); however, subsequent assessments using a Renilla reniformis luciferase reporter have failed to produce nonsense codon suppression activity. Thus, while PTC-124 is in clinical testing in patients with nonsense mutations within the CFTR or dystrophin genes, controversy surrounds its exact mechanism of action.
Uses
Nonsense mutations create a premature termination of mRNA translation and have been implicated in various genetic disorders, including muscular dystrophy and cystic fibrosis. PTC-124 is a nonaminoglycoside that has been reported to selectively induce ribosomes to read through premature nonsense stop signals on mRNA, thus allowing the production of full-length, functional proteins. In a mouse model of cystic fibrosis caused by nonsense mutations, PTC-124 treatment (60 mg/kg s.c. injection or 0.3-0.9 mg/ml orally) has been shown to restore cystic fibrosis transmembrane conductance regulator (CFTR) protein expression and function. The target activity of PTC-124 was initially evaluated by firefly luciferase reporter cell-based nonsense codon assay (IC50 = 7 nM); however, subsequent assessments using a Renilla reniformis luciferase reporter have failed to produce nonsense codon suppression activity. Thus, while PTC-124 is in clinical testing in patients with nonsense mutations within the CFTR or dystrophin genes, controversy surrounds its exact mechanism of action.[Cayman Chemical]
Uses
PTC-124 is a nonaminoglycoside that has been reported to induce ribosomes to read through premature nonsense stop signals on mRNA, allowing the production of full-length functional proteins,
Definition
ChEBI: 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic acid is a ring assembly and an oxadiazole.
brand name
Translarna?
Mechanism of action
The mechanism of action of Ataluren (PTC124) is to generate functionally normal myotonic dystrophy proteins by facilitating ribosomal read-through of nonsense mutations, thereby bypassing the pathogenic variant and continuing the translation process. Under normal conditions, ribosomes move along the mRNA that links amino acids into proteins until they reach the stop codon. When the ribosome encounters a premature termination codon (PTC) due to a nonsense mutation, eukaryotic release factors [eRF1 (green) and eRF3 (turquoise) complexed with GTP (yellow)] are recruited, and translation is terminated prematurely to produce the truncated protein. Ataluren is thought to interact with the ribosome to promote the recruitment of the proximity-recognition tRNAs, which in turn inhibits the nonsense mutation, allowing the PTC to be read through and synthesised. PTC to be read through and synthesise full-length proteins. The red amino acid on the near-recognition tRNA is incorporated into the read-through protein product. In the Ataluren-mediated nonsense repression model, the X on the tRNA indicates a mispairing at codon position three.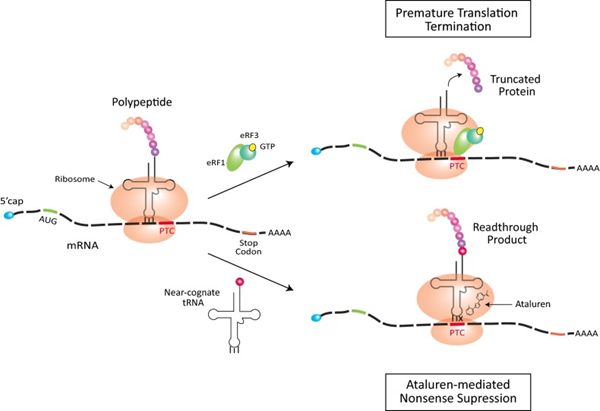
Side effects
The most common side effects of Ataluren include: vomiting, diarrhoea, nausea, headache, loss of appetite, epigastric pain and flatulence. children between the ages of 2-5 years are prone to tiredness and weakness, fever, ear infections and rash. Other side effects that may occur are high triglyceride levels, high blood pressure, cough, nosebleeds, blood in the urine, and pain in the extremities. Most of the above adverse reactions are mild to moderate. No treatment-related serious adverse reactions have been reported in clinical studies. However, there have been patients who discontinued treatment due to constipation and incapacitating adverse reactions. Therefore, seek prompt medical attention if serious adverse reactions occur.
Synthesis
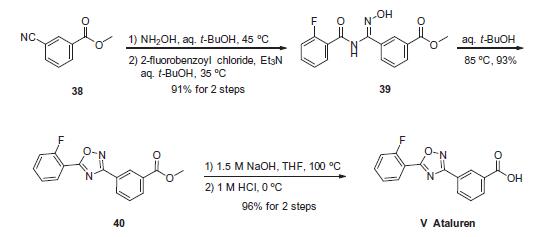
The sequence to construct ataluren, which was described by the authors at PTC Therapeutics, commenced with commercially available methyl 3-cyanobenzoate (38). This ester was exposed to hydroxylamine in aqueous tert-butanol and warmed gently until the reaction was deemed complete. Then this mixture was treated with 2-fluorobenzoyl chloride dropwise and subsequently triethylamine dropwise. To minimize exotherm and undesired side products, careful control of the addition of reagents was achieved through slow dropwise addition of these liquid reagents. Upon complete consumption of starting materials and formation of amidooxime 39, the aqueous reaction mixture was then heated to 85 ?? to facilitate 1,2,4-oxadiazole formation, resulting in the tricyclic ester 40 in excellent yield across the three steps. Finally, saponification of ester 40 through the use of sodium hydroxide followed by acidic quench gave ataluren (V) in 96% over the two-step sequence.
target
CFTR
Ataluren Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Famo Biotech Co Ltd | +86-36680037 +86-18550473860 | mayan@famobiotech.com;sales@famobiotech.com | China | 528 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 998 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +86-15350851019 +86-15383190639 | admin@86-ss.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
View Lastest Price from Ataluren manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Ataluren (PTC124)
775304-57-9
|
US $100.00-75.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-19 | Ataluren
775304-57-9
|
US $32.00-85.00 / mg | 99.84% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Ataluren
775304-57-9
|
US $0.00 / KG | 1KG | 98% HPLC | 5000kgs | shandong perfect biotechnology co.ltd |
-

- Ataluren (PTC124)
775304-57-9
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Ataluren
775304-57-9
- US $32.00-85.00 / mg
- 99.84%
- TargetMol Chemicals Inc.
-

- Ataluren
775304-57-9
- US $0.00 / KG
- 98% HPLC
- shandong perfect biotechnology co.ltd
775304-57-9(Ataluren)Related Search:
1of4