2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid
- CAS No.
- 331002-70-1
- Chemical Name:
- 2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid
- Synonyms
- RTHRCOIONCZINZ-JMIUGGIZSA-N;2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid;Benzoic acid, 2-chloro-5-[[5-[[5-(4,5-dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]-
- CBNumber:
- CB02518882
- Molecular Formula:
- C23H16ClN3O6S
- Molecular Weight:
- 497.91
- MDL Number:
- MOL File:
- 331002-70-1.mol
2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid Properties
| Boiling point | 696.8±65.0 °C(Predicted) |
|---|---|
| Density | 1.53±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solid |
| pka | 2.74±0.25(Predicted) |
| color | Brown |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501 | |||||||||
| NFPA 704 |
|
2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 21335 | PT1 ≥95% | 331002-70-1 | 1mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 21335 | PT1 ≥95% | 331002-70-1 | 5mg | $116 | 2024-03-01 | Buy |
| Cayman Chemical | 21335 | PT1 ≥95% | 331002-70-1 | 10mg | $191 | 2024-03-01 | Buy |
| Cayman Chemical | 21335 | PT1 ≥95% | 331002-70-1 | 25mg | $442 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | KIN0000873 | PT-1 95.00% | 331002-70-1 | 5MG | $504.35 | 2021-12-16 | Buy |
2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid Chemical Properties,Uses,Production
Biological Activity
pt 1 is a selective activator of ampk with ec50 value of 0.3 μm for ampk α1β1γ1 [1].amp-activated protein kinase (ampk) is serine/threonine protein kinase that involved in cellular energy homeostasis and acts as an energy sensor. ampk is a heterotrimer and increases atp generation [1].pt 1 is a selective ampk activator. pt1 activated human ampk α1394, ampk α2398 and ampk(α1β1γ1) with ec50 values of 8, 12 and 0.3 μm, respectively. pt1 exhibited maximum activity against ampk(α1β1γ1) at concentration up to 5 μm. pt1 exhibited high selectivity for ampk α catalytic subunit. pt1 activated truncated ampk α1 subunit proteins including 313-335 aa with ec50 values of 8 μm, which was autoinhibitory domain. in hela cells without lkb1, pt1 induced ampk and acc phosphorylation, which were independent of lkb1. in human hepatoma hepg2 cells, pt1 dose-dependently reduced triacylglycerol and cholesterol content and induced ampk and acc phosphorylation [1]. in incubated mouse muscle, pt-1 increased γ1-containing ampk activity and increased the ampk-dependent phosphorylation of ulk1 on ser555. however, in hek293 cells expressing human γ1-, γ2- or γ3-ampk, pt-1 activated them equally [2].
Enzyme inhibitor
This AMPK activator (FW = 497.91 g/mol; CAS 331002-70-1), systematically named 2-chloro-5-[[5-[[5- (4,5-dimethyl-2-nitrophenyl) -2- furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoic acid, targets AMP-activated protein kinase (EC50 = 0.3 μM) by antagonizing its built-in autoinhibition mechanism. PT1 dose-dependently activates AMPK α1394, α1335, α2398, and even the heterotrimer α1β1γ1 form. Based on the structure of PT1 docked to AMPK α1 subunit, it appears that PT1 interacts with Glu-96 and Lys-156 near the autoinhibitory domain, directly relieving autoinhibition. In studies using L6 myotubes, the phosphorylation of AMPK and its downstream substrate, acetyl-CoA carboxylase, were dose-dependently and time-dependently increased by PT1 without any change in cellular AMP:ATP concentration ratio.
References
[1]. pang t, zhang zs, gu m, et al. small molecule antagonizes autoinhibition and activates amp-activated protein kinase in cells. j biol chem, 2008, 283(23): 16051-16060.
[2]. jensen te, ross fa, kleinert m, et al. pt-1 selectively activates ampk-γ1 complexes in mouse skeletal muscle, but activates all three γ subunit complexes in cultured human cells by inhibiting the respiratory chain. biochem j, 2015, 467(3): 461-472.
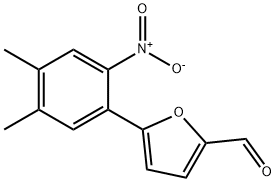
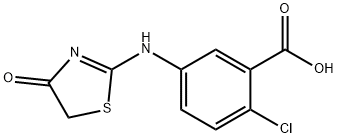

2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid Preparation Products And Raw materials
Raw materials
Preparation Products
2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8449 | 60 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9708 | 58 |
| BOC Sciences | -- | info@bocsci.com | USA | 0 | 65 |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 | sales@chemegen.com | China | 11289 | 58 |
| MedBioPharmaceutical Technology Inc | 021-69568360 18916172912 | order@med-bio.cn | China | 8141 | 58 |
| Changzhou Furuisi Biotechnology Co., Ltd | 0519-85524369 | 3477467573@qq.com | China | 8618 | 58 |
| ApexBio Technology | -- | sales@apexbt.com | United States | 6254 | 58 |





![2-Chloro-5-[[5-[[5-(4,5-Dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl]amino]benzoicacid Structure](CAS/GIF/331002-70-1.gif)