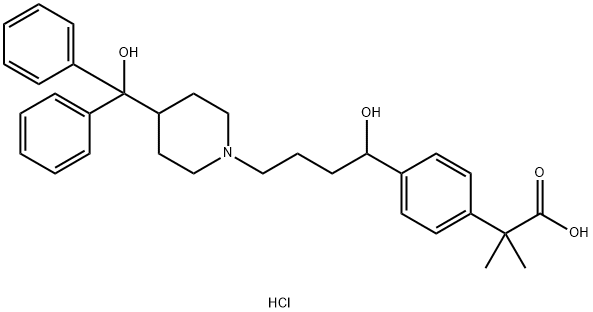
Fexofenadine hydrochloride
- CAS No.
- 153439-40-8
- Chemical Name:
- Fexofenadine hydrochloride
- Synonyms
- Fexo;Allegra;2-[4-[1-Hydroxy-4-[4-(hydroxy-diphenyl-methyl)-1-piperidyl]-butyl]phenyl]-2-methyl-propanoic acid hydrochloride;Telfast;Alernex;Feksine;Fexofen;Fexadyne;Vivafeks;MDL 16455A
- CBNumber:
- CB0256621
- Molecular Formula:
- C32H40ClNO4
- Molecular Weight:
- 538.13
- MDL Number:
- MFCD08064193
- MOL File:
- 153439-40-8.mol
- MSDS File:
- SDS
| Melting point | 148-150oC |
|---|---|
| RTECS | CY1633377 |
| storage temp. | 2-8°C |
| solubility | Soluble in Dimethyl sulfoxide (50 mM), and methanol. |
| form | powder |
| color | white to beige |
| Merck | 14,4068 |
| InChIKey | RRJFVPUCXDGFJB-UHFFFAOYSA-N |
| CAS DataBase Reference | 153439-40-8(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Allegra |
| FDA UNII | 2S068B75ZU |
| NCI Drug Dictionary | Allegra |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H360-H312 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P280-P302+P352-P312-P322-P363-P501 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| HS Code | 2933399090 |
Fexofenadine hydrochloride price More Price(51)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | F9427 | Fexofenadine hydrochloride >98% (HPLC) | 153439-40-8 | 10mg | $187 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1270377 | Fexofenadine hydrochloride United States Pharmacopeia (USP) Reference Standard | 153439-40-8 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | F0698 | Fexofenadine Hydrochloride >98.0%(HPLC)(T) | 153439-40-8 | 1g | $73 | 2024-03-01 | Buy |
| TCI Chemical | F0698 | Fexofenadine Hydrochloride >98.0%(HPLC)(T) | 153439-40-8 | 5g | $200 | 2024-03-01 | Buy |
| Alfa Aesar | J63262 | Fexofenadine hydrochloride | 153439-40-8 | 25mg | $108.65 | 2024-03-01 | Buy |
Fexofenadine hydrochloride Chemical Properties,Uses,Production
Description
Fexofenadine hydrochloride (trade name: Allegra) belongs to a second-generation antihistamine pharmaceutical drug. It is mainly used for the treatment of allergy symptoms including watery eyes, hay fever, nasal congestion, urticaria, runny nose, itching eyes/nose, sneezing, hives, and itching. It is a kind of selectively peripheral H1 blocker. Its mechanism of action in vivo is through preventing the histamine-mediated activation of the H1 receptors, thus alleviating the symptoms related to allergy. Compared to the first-generation antihistamine drug, it is less prone to penetrate the blood-brain barrier, reducing the possibility to cause side effects such as drowsiness.
Mechanism of Action
Fexofenadine hydrochloride is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine inhibited histamine release from peritoneal mast cells in rats. In laboratory animals, no anticholinergic, alpha1-adrenergic or beta-adrenergic-receptor blocking effects were observed. No sedative or other central nervous system effects were observed. Radiolabeled tissue distribution studies in rats indicated that fexofenadine does not cross the blood-brain barrier.
Biochem/physiol Actions
Fexofenadine is used in treating allergic rhinitis and chronic idiopathic urticaria. It may give relief in bothersome symptom and is a second-generation antihistamines. Fexofenadine provides better relief compared to other antihistamines for nasal congestion. It is the active metabolite of terfenadine and bring down allergen-induced symptoms.
Fexofenadine is a non-sedating H1 histamine receptor antagonist.
Indications
Seasonal Allergic Rhinitis
Fexofenadine hydrochloride(ALLEGRA) is indicated for the relief of symptoms associated with seasonal allergic rhinitis in adults and children 6 years of age and older. Symptoms treated effectively were sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes.
Chronic Idiopathic Urticaria
ALLEGRA is indicated for treatment of uncomplicated skin manifestations of chronic idiopathic urticaria in adults and children 6 years of age and older. It significantly reduces pruritus and the number of wheals.
CONTRAINDICATIONS
ALLEGRA is contraindicated in patients with known hypersensitivity to any of its ingredients.
Overdosage
Reports of fexofenadine hydrochloride overdose have been infrequent and contain limited information. However, dizziness, drowsiness, and dry mouth have been reported. Single doses of fexofenadine hydrochloride up to 800 mg (six normal volunteers at this dose level), and doses up to 690 mg twice daily for 1 month (three normal volunteers at this dose level) or 240 mg once daily for 1 year (234 normal volunteers at this dose level) were administered without the development of clinically significant adverse events as compared to placebo.
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended.
Hemodialysis did not effectively remove fexofenadine hydrochloride from blood (1.7% removed) following terfenadine administration.
No deaths occurred at oral doses of fexofenadine hydrochloride up to 5000 mg/kg in mice (110 times the maximum recommended daily oral dose in adults and 200 times the maximum recommended daily oral dose in children based on mg/m2 ) and up to 5000 mg/kg in rats (230 times the maximum recommended daily oral dose in adults and 400 times the maximum recommended daily oral dose in children based on mg/m2 ). Additionally, no clinical signs of toxicity or gross pathological findings were observed. In dogs, no evidence of toxicity was observed at oral doses up to 2000 mg/kg (300 times the maximum recommended daily oral dose in adults and 530 times the maximum recommended daily oral dose in children based on mg/m2 ).
References
http://www.rxlist.com/allegra-drug/consumer-uses.htm
https://en.wikipedia.org/wiki/Fexofenadine
Description
Fexofenadine is a histamine H1 receptor antagonist (Ki = 10 nM). It reverses contraction of isolated rat tracheal strips induced by acetyl-β-methylcholine . Fexofenadine inhibits expression of IL-8 in TNF-α-stimulated HCT116 and COLO 205 cells. Oral administration of fexofenadine (2 and 10 mg/kg) reduces severity of colitis and phospho-IκB kinase activation in a mouse model of colitis induced by dextran sulfate sodium (DSS; ).
Chemical Properties
Off-White Crystalline Solid
Originator
Alernex,Dabur Pharmaceuticals Ltd.,India
Uses
Fexofenadine is a non-sedating antihistamine that selectively antagonizes the histamine H1 receptor with a Ki value of 10 nM and exhibits anti-inflammatory effects. It is devoid of central nervous system effects in part because it is a good substrate for the P-glycoprotein efflux pump situated within the blood-brain barrier.[Cayman Chemical]
Uses
A metabolite of of terfenadine, a H1-Histamine receptor antagonist
Uses
The active metabolite of Terfenadine (T114500), a H1-histamine receptor antagonist.
Uses
Fexofenadine hydrochloride is used as an antihistamine that inhibits Cox-1, Cox-2, and is a histamine H1 receptor agonist.
Uses
Antihistaminic;histamine H1-receptor antagonist
Definition
ChEBI: Fexofenadine hydrochloride is a diarylmethane.
Manufacturing Process
Microbiological Preparation of Fexofenadine (Patent U.S. 6,558,931)
Ten 250 ml Erlenmeyer flasks containing 100 ml of medium D are seeded with
A. corymbifera LCP 63-1800. 50 mg of Terfenadine in 1 ml of ethanol is added
to each Erlenmeyer flask. The content of 10 Erlenmeyer flasks are filtered on
gauze (the supernatant has a pH equal to 8.0), and saturation with sodium
chloride is carried out for 2 hours (pH = 5-6), followed by extracting 3 times
with ethyl acetate and drying over magnesium sulfate. After evaporation
under reduced pressure, 409 mg of expected crude product is obtained (yield
= 77%, approximately 90% pure product). This product is purified on a
column of silica gel (230-400 mesh, 40 g of silica, diameter = 3 cm) eluting
with a methylene chloride/methanol/ammonium hydroxide mixture
(82.5:15:2.5). 312 mg of pure Fexofenadine is recovered (61.4%).
Synthesis of Fexofenadine (Patent U.S. No. 5,578,610
Aluminum chloride (44 g; 0.33 mol) was added in portions to a solution of
freshly distilled 4-chlorobutyryl chloride (17 mL; 0.15 mol) in 460 mL of
carbon disulfide at -10°C under a nitrogen atmosphere. The mixture was
stirred for 15 min, then the cooling bath was removed and the mixture was
allowed to warm to ambient temperature. The mixture was stirred then for 15
min more, then cooled again to -10°C and a solution of ethyl-α,α-
dimethylphenyl acetate (26.6 g; 0.14 mol) in 70 mL of carbon disulfide was
added dropwise. The mixture was stirred for 3 hours, then stirred overnight at
room temperature. The reaction mixture was partitioned between water and
CHCl3. The combined organic portions were dried over MgSO4, filtered and
concentrated in vacuo. The residue was dissolved in CH2Cl2 and filtered
through a plug of SiO2, eluting with 10% EtOAc in hexane. Yield of ethyl 3-
and 4-(4-chloro-1-oxobutyl)-α,α-dimethylphenylacetate 39.4 g (as a mixture
of aromatic regioisomers).
To a solution of 39.4 g of ethyl 3- and 4-(4-chloro-1-oxobutyl)-α,α-
dimethylphenylacetate dissolved in 800 mL of methanol and 200 mL of water
was added 40 g of NaOH. The mixture was refluxed for one hour. The cooled
mixture was then concentrated in vacuo. The concentrate was diluted with
water and washed with 2 portions of EtOAc. The aqueous layer was acidified
with concentrated HCl and extracted with 2 portions of EtOAc. The extracts
were dried over MgSO4, filtered, and concentrated in vacuo to afford 30.3 g of
crude product. The crude product was dissolved in 600 mL of EtOAc, 38 g of
cinchonidine was added, and the mixture was stirred overnight. The resulting
solids were filtered and washed with EtOAc and sucked dry under a rubber
dam to afford 25 g of a solid 4-(cyclopropyl-oxo-methyl)-α,α-
dimethylphenylacetic acid.
A solution of 10.5 g of 4-(cyclopropyl-oxo-methyl)-α,α-dimethylphenylacetic
acid in 250 mL of CH2Cl2 was cooled in an ice-MeOH bath and 25 g of
trimethylsilyliodide was then added via pipette. The mixture was stirred in the
ice bath for one hour, warmed to ambient temperature, and stirred for one
hour. A solution of aqueous sodium bisulfite was then added and the mixture
was stirred well. The phases were partitioned and the aqueous layer was
extracted with CH2Cl2. The combined organics were washed with saturated
aqueous NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford
12.6 g (77%) of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid.
To a solution of 12.6 g of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid
in 100 mL of ether cooled in an ice bath, was added 40 mL of ethereal CH2N2.
The mixture was stirred at 0°C for few minutes, then let stand for 2 hours. A
few drops of AcOH were added to decompose excess CH2N2, then the mixture
was filtered and stripped to afford 12.6 g (96%) of methyl 4-(4-iodo-1-
oxobutyl)-α,α-dimethylphenylacetate.
A solution of 12.6 g of methyl 4-(4-iodo-1-oxobutyl)-α,α-
dimethylphenylacetate in 500 mL of toluene in a one liter three neck flask was
added 8.8 g of 4-(α,α-diphenyl)piperidinemethanol and 23 g of K2CO3 and the
mixture was refluxed for 7 hours. The cooled reaction mixture was then
filtered and concentrated in vacuo. The residue was dissolved in ether and
treated with excess ethereal HCl. The mixture was then concentrated to a
solid. The solid was treated with EtOAc and collected by filtration. The product
was then partitioned between EtOAc and 2 N Na2CO3. The organics were dried
over MgSO4, filtered, and concentrated in vacuo to afford 13.5 g (79%) of
methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-oxobutyl]-α,α-
dimethylphenylacetate.
A solution of 13.5 g of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-
1-oxobutyl]-α,α-dimethylphenylacetate in 250 mL of methanol was cooled in
an ice-methanol bath and 1.8 g of NaBH4 was added in portions. After 1 hour,
the mixture was concentrated to a solid. The residue was partitioned between
EtOAc and saturated aqueous NaHCO3. The aqueous portion was extracted
with EtOAc. The combined organics were washed with saturated aqueous
NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford 9.5 g
(70%) of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-
hydroxybutyl]-α,α-dimethylphenylacetate as a foam.
To a solution of 9.5 g of methyl-4-[4-[4-(hydroxydiphenylmethyl)-1-
piperidinyl]-1-hydroxybutyl]-α,α-dimethylphenylacetate in 300 mL of methanol
and 150 mL of water was added 10 g of NaOH. The mixture was refluxed for 1
hour, then cooled. The methanol was removed in vacuo. The concentrate was
diluted with water and CHCl3 and the pH adjusted to approximately 5.5 to 6.0.
The phases were separated and the aqueous phase was extracted with CHCl3.
The combined organics were dried over MgSO4, filtered, and stripped to afford
9.0 g of crude product. The crude product was dissolved in CH2Cl2 and
chromatographed on Davisil Grade 633 SiO2 eluting with a gradient of CHCl3,
to 10% of methanol in CHCl3, to 25% of methanol in CHCl3. The product was
concentrated to afford 5.2 g of white crystals of 4-[4-[4-
(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-α,α-dimethylphenylacetic acid (Fexofenadine).
In practice it is usually used as hydrochloride salt.
Therapeutic Function
Antihistaminic
General Description
Fexofenadine hydrochloride, (±)-4-[1-hydroxy-4-[4-(hydroxyldiphenylmethyl)- 1-piperinyl]butyl-α,α-dimethylbenzeneacetic acid (Allegra), occurs as a white to off-white crystalline powder that is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane.
This compound is marketed as a racemate and exists as a zwitterion in aqueous media at physiological pH. Fexofenadine is a primary oxidative metabolite of terfenadine. Terfenadine was developed during a search for new butyrophenone antipsychotic drugs as evidenced by the presence of the N-phenylbutanol substituent. It also contains a diphenylmethyl-piperidine moiety analogous to that found in the piperazine antihistamines.
Biological Activity
Fexofenadine hydrochloride is a selective histamine H1 receptor antagonist (pKi = 8.1). Active metabolite of Terfenadine that displays non-sedating antiallergic effects. Fexofenadine hydrochloride counteracts histamine mediated immune suppression and improves immunotherapy response.
Biochem/physiol Actions
Fexofenadine is a non-sedating H1 histamine receptor antagonist.
Clinical Use
Antihistamine:Symptomatic relief of rhinitis and urticaria
Drug interactions
Potentially hazardous interactions with other drugs Antibacterials: effects possibly reduced by rifampicin. Antivirals: concentration possibly increased by ritonavir. Aluminium/magnesium containing antacids: reduced absorption - avoid for 2 hours.
Metabolism
Fexofenadine undergoes negligible metabolism (hepatic or non-hepatic); about 5% of the total dose is metabolised, mostly by the intestinal mucosa, with 0.5-1.5% of the dose undergoing hepatic biotransformation by the cytochrome P450 system. The major route of elimination is believed to be via biliary excretion while up to 10% of ingested dose is excreted unchanged through the urine.
Fexofenadine hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 | mack@tongyon.com | China | 2994 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 444 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +86-15350851019 +86-15383190639 | admin@86-ss.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
View Lastest Price from Fexofenadine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Fexofenadine hydrochloride
153439-40-8
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%-102%; USP | 500KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-22 | Fexofenadine hydrochloride
153439-40-8
|
US $100.00-75.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-19 | Fexofenadine hydrochloride
153439-40-8
|
US $97.00-71.00 / mg | ≥95% | 10g | TargetMol Chemicals Inc. |
-

- Fexofenadine hydrochloride
153439-40-8
- US $0.00-0.00 / Kg/Drum
- 98%-102%; USP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Fexofenadine hydrochloride
153439-40-8
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Fexofenadine hydrochloride
153439-40-8
- US $97.00-71.00 / mg
- ≥95%
- TargetMol Chemicals Inc.
153439-40-8(Fexofenadine hydrochloride )Related Search:
1of4