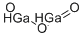Gallium(III) oxide
- CAS No.
- 12024-21-4
- Chemical Name:
- Gallium(III) oxide
- Synonyms
- GALLIUM OXIDE;Ga2-O3;Gallia;β-Ga2O3;Gallic oxide;ium(III) oxide;galliumtrioxide;Gallium(Ⅲ) oxide;GALLIUM(+3)OXIDE;GalliuM(Ⅵ) oxide
- CBNumber:
- CB0268996
- Molecular Formula:
- Ga2O3
Lewis structure

- Molecular Weight:
- 187.44
- MDL Number:
- MFCD00011020
- MOL File:
- 12024-21-4.mol
| Melting point | 1740 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | 6.44 g/mL at 25 °C | ||||||||||||||
| refractive index | 1.92 | ||||||||||||||
| storage temp. | Sealed in dry,Room Temperature | ||||||||||||||
| solubility | soluble in hot acid solutions | ||||||||||||||
| form | Powder or Lump | ||||||||||||||
| Specific Gravity | 6.44 | ||||||||||||||
| color | White | ||||||||||||||
| Water Solubility | Soluble in acids. Insoluble in water | ||||||||||||||
| Crystal Structure | Corundum type | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Merck | 14,4346 | ||||||||||||||
| Space group | R3c | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Stability | Stable. Incompatible with magnesium. | ||||||||||||||
| InChIKey | QZQVBEXLDFYHSR-UHFFFAOYSA-N | ||||||||||||||
| CAS DataBase Reference | 12024-21-4(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1 | ||||||||||||||
| FDA UNII | 46F059V66A | ||||||||||||||
| EPA Substance Registry System | Gallium trioxide (12024-21-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | LW9650000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28259090 | |||||||||
| Toxicity | mammal (species unspecified),LDLo,intraperitoneal,10gm/kg (10000mg/kg),BEHAVIORAL: EXCITEMENTLUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONBEHAVIORAL: ATAXIA,Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(6), Pg. 58, 1987. | |||||||||
| NFPA 704 |
|
Gallium(III) oxide price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 215066 | Gallium(III) oxide ≥99.99% trace metals basis | 12024-21-4 | 10g | $134 | 2024-03-01 | Buy |
| Sigma-Aldrich | 215066 | Gallium(III) oxide ≥99.99% trace metals basis | 12024-21-4 | 50g | $477 | 2024-03-01 | Buy |
| Alfa Aesar | 010508 | Gallium(III) oxide, 99.999% (metals basis) | 12024-21-4 | 25g | $318 | 2024-03-01 | Buy |
| Alfa Aesar | 010508 | Gallium(III) oxide, 99.999% (metals basis) | 12024-21-4 | 5g | $85.5 | 2023-06-20 | Buy |
| Strem Chemicals | 93-3106 | Gallium(III) oxide (99.998%-Ga) PURATREM | 12024-21-4 | 5g | $61 | 2024-03-01 | Buy |
Gallium(III) oxide Chemical Properties,Uses,Production
Physical properties
White crystals; exists in three crystalline modifications: alpha-, beta-, and gamma-Ga2O3; while the alpha-form is analogous to the corundum form of alumina, the beta-Ga2O3 is isomorphous with theta-alumina; alpha-form converts to beta-modification on calcination at high temperatures (600°C); gamma form is stable at low temperatures; density 6.44 g/cm3 (alpha-Ga2O3), 5.88 g/cm3 (betaGa2O3); melts at 1,725°C; soluble in most acids.

Gallium(III) oxide is used in the manufacture of gadolinium gallium garnet as a substrate in bubble domain memories; these are compact devices not requiring the storage power demanded by other memory devices. Gallium(III) oxide is starting material for the preparation of Sr2CuGaO3S, an example of a rare square pyramidal gallium.
Preparation
Gallium(III) oxide is precipitated in hydrated form upon neutralization of acidic or basic solution of gallium salt. Also, it is prepared by thermal decomposition of gallium salts. Gallium oxide hydroxide, GaOOH [20665-525] on calcinations at high temperatures yields betaGa2O3.
Reactions
Gallium(III) oxide is reduced to gallium suboxide, Ga2O [12024-20-3] by common reducing agents. Also, heating the sesquioxide with gallium metal yields gallium suboxide. Heating with magnesium reduces the oxide to elemental form in a violent reaction:
Ga2O3 + 3Mg →2Ga + 3MgO
Heating with mineral acids yields corresponding gallium salts. When heated with a mixture of hydrogen and arsenic vapors at 600°C, gallium arsenide, GaAs is produced. When heated with alkali metal oxide at 1,000°C, alkali metal gallates, such as K2Ga2O6 are formed.
Description
Gallium(III) oxide (Ga2O3) is a wide band gap semiconductor that belongs to a family of transparent semiconducting oxides (TSO). It can form different polymorphs such as α-,β-, γ-, δ-, and ε-. Polycrystalline and nanocrystalline Ga2O3 can be prepared using several methods such as chemical vapor deposition, thermal vaporization, and sublimation, molecular beam epitaxy, melt growth, etc. It is widely used as a functional material in various applications including optoelectronics, chemical sensors, catalysis, semiconductor devices, field-effect transistors, and many others.
Chemical Properties
white odourless powder
Uses
Gallium(III) oxide is used in vacuum deposition. It is useful for making semiconductor devices, gallium-alumina catalyst, gas sensors, luminescent phosphors and dielectric coatings of solar cells. It shows potential for developing deep-ultraviolet TCOs (Transparent Conductive Oxides) and transparent electrodes for ultraviolet optoelectronic devices. Recent studies report that gallium oxide can be a strong contender for power electronic devices for example, in ultrahigh-voltage power switching applications. Films made of gallium oxide have gained commercial interest owing to their gas sensitive characteristics, and glasses made with gallium oxide are the preferred optical materials for use in advanced technologies.
Uses
In semiconductors; gas sensing; catalysis. Nanostructures as blue and UV light emitters in optoelectronic device applications.
Gallium(III) oxide has been reported in applications in lasers, phosphors, and luminescent materials. Monoclinic ß-Ga2O3 is used in gas sensors and luminescent phosphors and can be applied to dielectric coatings for solar cells. This stable oxide has also shown potential for deep-ultraviolet transparent conductive oxides, and transistor applications. Thin Ga2O3 films are of commercial interest as gas sensitive materials. ß-Gallium(III) oxide is used in the production of Ga2O3-Al2O3 catalyst.
Production Methods
Gallium oxide is formed during the annealing or heating of semiconductors containing gallium in the microelectronics industry.
Application
Gallium(III) oxide is widely used as a host material for the fabrication of electroluminescent devices. For example, europium-doped Ga2O3 thin films can be used as a light-emitting layer to fabricate an optically transparent electroluminescent device.
Due to its distinct optical and electrical properties like moderate conductivity and high laser damage threshold, Ga2O3 can be used in laser-driven electron accelerators, low-loss plasmonics, and Si-based dielectric laser accelerators.
It can also be used as an effective catalyst for the dehydrogenation of propane to propene.
Starting material for the preparation of Sr2CuGaO3S, an example of a rare square pyramidal gallium.
Gallium(III) oxide is used in spectroscopic analysis and in preparing gallium arsenide for making semiconductors.
General Description
Odorless fine white powder. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
GALLIUM(III) OXIDE may react with acids. Heating (to 1292° F) with magnesium causes a violent reduction to metallic slate.
Health Hazard
SYMPTOMS: Symptoms of exposure to GALLIUM(III) OXIDE include reduction of red blood cells and platelets, anemia, skin rash and nausea.
Fire Hazard
Flash point data for GALLIUM(III) OXIDE are not available; however, GALLIUM(III) OXIDE is probably combustible.
References
Highly selective and rapid enrichment of phosphorylated peptides using gallium oxide‐coated magnetic microspheres for MALDI‐TOF‐MS and nano‐LC‐ESI‐MS/MS/MS analysis DOI:10.1002/PMIC.200700454
Development of gallium oxide power devices (Phys. Status Solidi A 1∕2014) DOI:10.1002/PSSA.201470201
Synthesis and characterization of morphologically different high purity gallium oxide nanopowders DOI:10.1007/S10853-007-1869-2
Structure, Morphology, and Optical Properties of Amorphous and Nanocrystalline Gallium Oxide Thin Films DOI:10.1021/JP311300E
Electrical and optical properties of deep ultraviolet transparent conductive Ga2O3/ITO films by magnetron sputtering DOI:10.1088/1674-4926/31/10/103001
Patty's Toxicology, 6 Volume Set, 6th Edition
Gallium(III) oxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 2904 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20285 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
View Lastest Price from Gallium(III) oxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-02 | Gallium(III) oxide
12024-21-4
|
US $9.90 / KG | 1KG | 99% | 5tons | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-11-14 | Gallium(III) oxide
12024-21-4
|
US $267.00 / KG | 1KG | 98% | 1-20mt | Baoji Guokang Bio-Technology Co., Ltd. | |
 |
2024-09-06 | Gallium(III) oxide
12024-21-4
|
US $6.00 / kg | 1kg | 0.99 | 10000 | Hebei Fengjia New Material Co., Ltd |
-

- Gallium(III) oxide
12024-21-4
- US $9.90 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Gallium(III) oxide
12024-21-4
- US $267.00 / KG
- 98%
- Baoji Guokang Bio-Technology Co., Ltd.
-

- Gallium(III) oxide
12024-21-4
- US $6.00 / kg
- 0.99
- Hebei Fengjia New Material Co., Ltd