ChemicalBook >> CAS DataBase List >>O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE
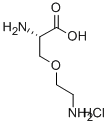
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE
- CAS No.
- 118021-35-5
- Chemical Name:
- O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE
- Synonyms
- L-4-OXALYSINE HCL;H-SER(ETNH2)-OH HCL;H-LYS[*4(<-O)]-OH HCL;4-OXY-L-LYSINE HYDROCHLORIDE;L-4-Oxalysine (hydrochloride);O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE;O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE USP/EP/BP;(2S)-2-amino-3-(2-aminoethoxy)propanoicacid,hydrochloride;(S)-(+)-2-AMINO-3-(2-AMINOETHOXY)PROPANOIC ACID, MONOHYDROCHLORIDE;(S)-(+)-2-AMINO-3-(2-AMINOETHOXY)-PROPAN OIC ACID HYDROCHLORIDE, 98%
- CBNumber:
- CB0297079
- Molecular Formula:
- C5H13ClN2O3
- Molecular Weight:
- 184.62
- MDL Number:
- MFCD01631149
- MOL File:
- 118021-35-5.mol
- MSDS File:
- SDS
Last updated:2024-07-02 08:54:57
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H335-H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ChemScene | CS-7133 | L-4-Oxalysinehydrochloride 97.10% | 118021-35-5 | 1mg | $104 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | AAA0007225 | O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE 95.00% | 118021-35-5 | 5MG | $503.66 | 2021-12-16 | Buy |
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE Chemical Properties,Uses,Production
Biological Activity
L-4-Oxalysine hydrochloride is a natural substance isolated from the culture medium of Streptomyces in China and has anticancer activity.
in vitro
L-4-oxalysine functionally antagonizes the a-fetoprotein-induced suppression of the mitogen- and one-way mixed lymphocyte culture-induced proliferation of spleen lymphocytes and interleukin-6 production by these cells in mice bearing the hepatoma-22 tumor.
in vivo
L-4-oxalysine inhibits the proliferation of some mouse implanted tumors and pulmonary metastasis of mouse Lewis lung carcinoma.
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE Suppliers
Global( 24)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22783 | 58 |
| BOC Sciences | 16314854226; +16314854226 | inquiry@bocsci.com | United States | 19741 | 58 |
| Changzhou Guiding Bio-Tech. Co.,Ltd. | +86-519-519-86686860 +8618795626222 | ning@guiding-bio.com | China | 299 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19908 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52924 | 58 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4861 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 18892239720 | psaitong@jm-bio.com | China | 12306 | 58 |
| Fan De(Beijing) Biotechnology Co., Ltd. | 15911056312 | liming@bio-fount.com | China | 9729 | 58 |
118021-35-5(O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE)Related Search:
H-SER(ETNH2)-OH HCL
H-LYS[*4(<-O)]-OH HCL
L-4-OXALYSINE HCL
(S)-(+)-2-AMINO-3-(2-AMINOETHOXY)PROPANOIC ACID, MONOHYDROCHLORIDE
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE
L-4-Oxalysine (hydrochloride)
(S)-(+)-2-AMINO-3-(2-AMINOETHOXY)-PROPAN OIC ACID HYDROCHLORIDE, 98%
4-OXY-L-LYSINE HYDROCHLORIDE
(2S)-2-amino-3-(2-aminoethoxy)propanoicacid,hydrochloride
O-(2-AMINOETHYL)-L-SERINE HYDROCHLORIDE USP/EP/BP
118021-35-5
C5H13ClN2O3
H2NCH2CH2OCH2CHNH2CO2HHCl
C5H12N2O3HCl
Peptide Synthesis
Others
Unnatural Amino Acid Derivatives
Specialty Synthesis
Others
Peptide Synthesis
Unnatural Amino Acid Derivatives