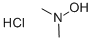N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE
- CAS No.
- 16645-06-0
- Chemical Name:
- N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE
- Synonyms
- DIMETHYLHYDROXYLAMINEHYDROCHLORIDE;N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE;N-hydroxy-N,N-dimethylamine hydrochloride;N,N-Dimethylhydroxylamine hydrochloride 99%
- CBNumber:
- CB0305325
- Molecular Formula:
- C2H8ClNO
- Molecular Weight:
- 97.54
- MDL Number:
- MFCD00012600
- MOL File:
- 16645-06-0.mol
- MSDS File:
- SDS
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 221457 | N,N-Dimethylhydroxylamine hydrochloride 99% | 16645-06-0 | 5g | $339 | 2024-03-01 | Buy |
| Sigma-Aldrich | 221457 | N,N-Dimethylhydroxylamine hydrochloride 99% | 16645-06-0 | 25g | $723 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | CHM0124736 | N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE 95.00% | 16645-06-0 | 5G | $923.51 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0124736 | N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE 95.00% | 16645-06-0 | 25G | $1609.92 | 2021-12-16 | Buy |
| AHH | MT-19566 | N,N-Dimethylhydroxylamine hydrochloride 98% | 16645-06-0 | 25g | $306 | 2021-12-16 | Buy |
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE Chemical Properties,Uses,Production
Uses
N,N-Dimethylhydroxylamine hydrochloride was used in the synthesis of 4,4-dimethyl-2,5,5-triphenyl-l.3-dioxa-4-azonia-2-bora-5-boratacyclopentane. It was also used as a polymer-chain terminator.
Preparation
(a) Preparation of N,N-Dimethyl~(a-phenylethyl)amine Oxide. To a flask containing 11.0 g (0.074 mole) of N,N-dimethyl(a-phenylethyl)amine is added dropwise 18.3 g (0.19 mole) of 35% aqueous hydrogen peroxide, and the mixture is stirred for 11 hr at room temperature. The excess hydrogen peroxide is decomposed by stirring the mixture with 8 sqcm2 clean platinum foil until the evolution of oxygen ceases. Most of the water is removed by distillation at 35 mm Hg, at a bath temperature of 45-55°C. The material that condenses in the dry-ice trichloroethylene trap is saved for recovery in Procedure (b). The residual syrup is diluted with 25 ml of absolute ethanol, and the process is repeated twice while a capillary nitrogen ebulliator is used. The resulting amine oxide is a viscous syrup and has a slight odor of styrene.
(b) Decomposition of N,N-Dimethyl-(a-phenylethyl)amine Oxide to Ν,Ν-Dimethylhydroxylamine. A 100 ml round bottom flask equipped with a capillary nitrogen inlet, and containing the preceding amine oxide is connected by a large-diameter tube to a condenser set for distillation with two receivers. Each receiver is cooled in a mixture of dry ice and trichloroethylene. The system is evacuated to 5 mm, and the flask is immersed in an oil bath at 85°C. The bath temperature is raised slowly to 115°C over a 35-min period, in which time most of the material distilled over. The bath temperature is finally raised to 150°C, in order to complete the (decomposition) distillation process. The residue remaining averages 0.35 g. The distillate in the dry-ice trap here and from Procedure (a) are combined, pentane is added as a solvent, and the mixture is washed with dilute hydrochloric acid. The acid solution is extracted with pentane, the latter washed with water until neutral.
The product is obtained from the combined aqueous layers containing hydrochloric acid by concentration under reduced pressure, to give a crystalline residue. The latter, upon drying in a vacuum desiccator over P2O5 averages 6.73 g (94% yield), m.p. 101-101°C (sealed capillary). Recrys-tallization several times from a mixture of absolute ethanol and ether gives white, hygroscopic needles, m.p. 106.5-109°C (sealed capillary).
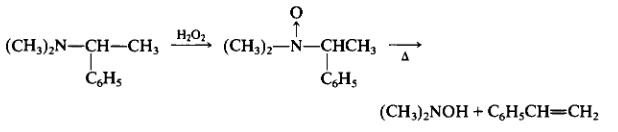
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10246 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Henan Fangding Technology Co., LTD | 15670369782 15670369782 | 15670369782@163.com | China | 9837 | 58 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44700 | 61 |
| Syntechem Co.,Ltd | info@syntechem.com | China | 12990 | 57 | |
| Bide Pharmatech Ltd. | 400-1647117 15221909166 | product02@bidepharm.com | China | 41438 | 60 |
| Shanghai Macklin Biochemical Co.,Ltd. | 15221275939 15221275939 | shenlinxing@macklin.cn | China | 15885 | 55 |
| Jiangsu Aikon Biopharmaceutical R&D co.,Ltd. | 025-66113011 18112977050 | cb6@aikonchem.com | China | 16687 | 50 |
16645-06-0(N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE)Related Search:
1of4