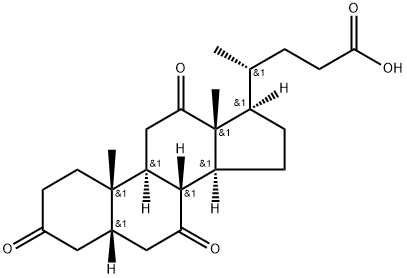
Dehydrocholic acid
- CAS No.
- 81-23-2
- Chemical Name:
- Dehydrocholic acid
- Synonyms
- DHC;dehydrocholic;Acolen;Atrocholin;Dehydrocholate;3,7,12-trioxo-5β-cholanic acid;5β-CHOLANIC acid-3, 7, 12-TRIONE;Dee-Co;Biochol;Dehycol
- CBNumber:
- CB0704485
- Molecular Formula:
- C24H34O5
- Molecular Weight:
- 402.53
- MDL Number:
- MFCD00066410
- MOL File:
- 81-23-2.mol
- MSDS File:
- SDS
| Melting point | 238-240 °C |
|---|---|
| alpha | 29 º (c=2, dioxane) |
| Boiling point | 444.65°C (rough estimate) |
| Density | 1.0687 (rough estimate) |
| vapor pressure | 0.013Pa at 20℃ |
| refractive index | 30.5 ° (C=2, Dioxane) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | ethanol: 10 mg/mL |
| form | Solid |
| pka | pKa 5.12(H2O t = 20 c > CMC) (Uncertain) |
| color | White to Off-White |
| Water Solubility | 65mg/L(30 ºC) |
| Merck | 14,2868 |
| BRN | 3226734 |
| LogP | 1.64 at 22℃ and pH5.7 |
| Surface tension | 56.9mN/m at 63mg/L and 20℃ |
| CAS DataBase Reference | 81-23-2(CAS DataBase Reference) |
| FDA 21 CFR | 310.545 |
| FDA UNII | NH5000009I |
| NIST Chemistry Reference | Dehydrocholic acid(81-23-2) |
| EPA Substance Registry System | Cholan-24-oic acid, 3,7,12-trioxo-, (5.beta.)- (81-23-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H303 | |||||||||
| Precautionary statements | P270-P301+P312-P403-P501c | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | FZ2300000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2918300000 | |||||||||
| NFPA 704 |
|
Dehydrocholic acid price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1166502 | Dehydrocholic acid United States Pharmacopeia (USP) Reference Standard | 81-23-2 | 200mg | $164.8 | 2024-03-01 | Buy |
| Cayman Chemical | 29176 | Dehydrocholic Acid | 81-23-2 | 1g | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 29176 | Dehydrocholic Acid | 81-23-2 | 5g | $93 | 2024-03-01 | Buy |
| TRC | D229340 | Dehydrocholic acid | 81-23-2 | 50g | $100 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD11044 | Dehydrocholic acid | 81-23-2 | 50g | $135.5 | 2021-12-16 | Buy |
Dehydrocholic acid Chemical Properties,Uses,Production
Description
Dehydrocholic acid is a semisynthetic bile acid, which is made by the oxidation of cholic acid by chromic acid. It can increase the output of bile by the liver and the filling of the gallbladder. Dehydrocholic acid aids the digestion of fats and increases absorption of fat soluble vitamins.
It is used as a gastrointestinal agent that stimulates the flow of bile into the duodenum (cholagogue) or stimulate the production of bile by the liver (choleretic). It is also used as laxative to relief constipation, diuretic, and a diagnostic aid.
References
[1] Giancarlo Cravotto, Arianna Binello, Luisa Boffa, Ornelio Rosati, Marco Boccalini, Stefano Chimichi (2006) Regio- and stereoselective reductions of dehydrocholic acid, Steroids, 469-475
[2] https://pubchem.ncbi.nlm.nih.gov/compound/dehydrocholic_acid
Chemical Properties
white to off-white amorphous powder
Originator
Dehydrocholic Acid,New Zealand Pharmaceuticals Limited (NZP)
Uses
antibacterial
Uses
Dehydrocholic Acid is a derivative of Cholic Acid (C432600). a choleretic produced by, and isolated from liver cells.
Definition
ChEBI: 3,7,12-trioxo-5beta-cholanic acid is an oxo-5beta-cholanic acid in which three oxo substituents are located at positions 3, 7 and 12 on the cholanic acid skeleton. It has a role as a gastrointestinal drug. It is an oxo-5beta-cholanic acid, a 7-oxo steroid, a 12-oxo steroid and a 3-oxo-5beta-steroid. It is a conjugate acid of a 3,7,12-trioxo-5beta-cholan-24-oate.
Manufacturing Process
A.) Oxidation of cholic acid:
A solution, consisting of 15.40 g of cholic acid and 18.75 g of anhydrous
sodium acetate in a solvent mixture of 20 ml of ethyl acetate, 30 ml of glacial
acetic acid, and 30 ml of water, was prepared. This solution was cooled to
20°C. Chlorine gas was bubbled into the solution with vigorous stirring while
the reaction temperature was maintained at 20°C. The chlorine was delivered
at a constant rate of about 2.5 g per hour over a 4-hour period. The total
amount of chlorine gas was 9.80 g which corresponds to about 3.68 moles per
mole of cholic acid, or approximately a 23% excess. The solution temperature
was maintained in the range of 16° to 20°C during the entire addition of
chlorine. Initially the cholic acid solution was very dark-colored. As the
reaction progressed, the solution became pale yellow and a precipitate of
sodium chloride deposited. A considerable amount of product and sodium
chloride precipitated during the latter stages of the reaction so that the final
reaction mixture was a heavy slurry which was difficult to stir. After the
addition of chlorine was complete, the slurry was aged one hour with stirring
at 20°C. The excess chlorine was then discharged by dropwise addition of
10% aqueous sodium sulfite until the solution gave a negative test to starchiodide paper. The semi-crystalline slurry was then diluted with water to raise
the total volume to 225 ml. The water was added dropwise with stirring over
a 1-hour period. The ethyl acetate was then distilled off at 65-88°C. The
resulting crystalline slurry was cooled to below 70°C and filtered through a
sintered-glass funnel of medium porosity. The filter cake was washed until the
filtrate gave a negative halide test with silver nitrate solution and then was
sucked partially dry on the funnel. Drying was completed in a drier at 110°C
for 3 hours. The product was crude pale tan dehydrocholic acid. Yield 14.3
(95%); M.P. 225-231°C.
B.) Purification of dehydrocholic acid:
To a chromatographic column, packed with 6.67 g of charcoal ("Nuchar C")
with layers of sea sand at either end, 75 ml of acetone was added to wet the
carbon. The column was heated to 40°C, and 25 ml of acetone was drained
off. A solution of 20 g of dry crude dehydrocholic acid in 500 ml of acetone
was poured into a reservoir atop the column and maintained in this reservoir
at 40°C. This solution was then allowed to drop through the column at a
constant rate over a 3-hour period. The column was then washed with 250 ml
of acetone flowing through the column at a constant rate over a 1-hour-period
at 40°C. The column effluent and wash acetone were combined and
concentrated to a residual volume of about 100 ml which resulted in the
formation of a thick slurry. The slurry was cooled with stirring at 0° to 5°C
and aged for 30 min at this temperature. The slurry was filtered and the filter
cake washed with cold acetone. The filter cake of U.S.P. dehydrocholic acid
was sucked partially dry on the filter and then dried at 110°C for 3 hours.
Yield 15 g to 17 g (75% to 85%).
A second crop of crystals was obtained from the combined filtrate and wash
liquid from the first crop filtration. This mixture, which initially had a volume
of about 100 ml, was concentrated to 20 ml. 10 ml of water was added to the
solution and 10 ml of acetone mixed with a small amount of water distilled off.
The residual thick slurry of dehydrocholic acid was cooled to 0-5°C, aged at this temperature with stirring for 30 min, and filtered. The filter cake was
washed with acetone at 0°C, partially dried by suction on the filter, and then
dried for three hours at 110°C. Yield 1 to 2 g (5% to 10%).
brand name
Decholin Sodium (Bayer).
Therapeutic Function
Choleretic, Diuretic, Diagnostic aid
Biochem/physiol Actions
Dehydrocholic acid is an oxidation product of cholic acid by chromic acid that is not present in physiological conditions. When used in animal experiments, it stimulates bile secretion.
Dehydrocholic acid Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5978 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | ada@sh-teruiop.com | China | 875 | 58 | |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 467 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
View Lastest Price from Dehydrocholic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Dehydrocholic acid
81-23-2
|
US $0.00 / KG | 1KG | 98%min | 500KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-22 | Dehydrocholic Acid
81-23-2
|
US $0.00-0.00 / kg | 1kg | 99% | 20tons | Sinoway Industrial co., ltd. | |
 |
2024-11-19 | Dehydrocholic acid
81-23-2
|
US $46.00 / mg | 95.14% | 10g | TargetMol Chemicals Inc. |
-

- Dehydrocholic acid
81-23-2
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Dehydrocholic Acid
81-23-2
- US $0.00-0.00 / kg
- 99%
- Sinoway Industrial co., ltd.
-

- Dehydrocholic acid
81-23-2
- US $46.00 / mg
- 95.14%
- TargetMol Chemicals Inc.
81-23-2(Dehydrocholic acid)Related Search:
1of4