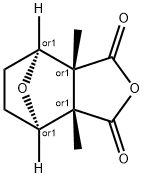
Cantharidin
- CAS No.
- 56-25-7
- Chemical Name:
- Cantharidin
- Synonyms
- Cantharone;cantharides;Canthari;7aalpha)-t;Antharidin;Kantaridin;CANTHARIDIN;Kantharidin;7aalpha)-bet;Cantharidine
- CBNumber:
- CB0710248
- Molecular Formula:
- C10H12O4
- Molecular Weight:
- 196.2
- MDL Number:
- MFCD00134968
- MOL File:
- 56-25-7.mol
| Melting point | 215-217 °C (lit.) |
|---|---|
| Boiling point | 253.08°C (rough estimate) |
| Density | 1.0357 (rough estimate) |
| refractive index | 1.4699 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (25 mg/ml warm), acetone (8 mg/ml), ethanol (8 mg/ml warm), chloroform (1:65), ether (1:560), and ethyl acetate (1:150). |
| form | solution |
| color | white |
| biological source | insect |
| Water Solubility | 30mg/L(20 ºC) |
| Merck | 14,1752 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 weeks. |
| InChIKey | DHZBEENLJMYSHQ-XCVPVQRUSA-N |
| LogP | -0.409 (est) |
| CAS DataBase Reference | 56-25-7(CAS DataBase Reference) |
| EWG's Food Scores | 1-3 |
| FDA UNII | IGL471WQ8P |
| NIST Chemistry Reference | Cantharidin(56-25-7) |
| IARC | 3 (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Cantharidin (56-25-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P270-P301+P310-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | T+,T | |||||||||
| Risk Statements | 28-36/37/38-38-37-36-23/24/25 | |||||||||
| Safety Statements | 53-45-36/37/39 | |||||||||
| RIDADR | UN 2811 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RN8575000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29329990 | |||||||||
| Hazardous Substances Data | 56-25-7(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Cantharidin price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C7632 | Cantharidin | 56-25-7 | 25mg | $87.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | C7632 | Cantharidin | 56-25-7 | 100mg | $154.8 | 2024-03-01 | Buy |
| TCI Chemical | C0019 | Cantharidin >98.0%(GC) | 56-25-7 | 25mg | $63 | 2024-03-01 | Buy |
| TCI Chemical | C0019 | Cantharidin >98.0%(GC) | 56-25-7 | 100mg | $172 | 2024-03-01 | Buy |
| Alfa Aesar | J61801 | Cantharidin, 98% | 56-25-7 | 100mg | $284 | 2024-03-01 | Buy |
Cantharidin Chemical Properties,Uses,Production
Description
Cantharidin is a natural toxicant of blister beetles. Cantharidin has been used as a medicinal agent for over 2000 years and is listed as a drug under the name of Mylabris in the medical monograph Materia Medica published in 77 AD. Some of the most ancient Chinese prescriptions (306–168 BC) refer to the use of Mylabris for the treatment of furuncles and piles.
Cantharidin can be used for topically (0.7%) treatment of warts, in which the skin under the wart blisters, thereby, lifting the wart off the skin. It is used to treat molluscum contagiosum. Cantharidin is a strong inhibitor of protein phosphatases types 1 (PP1) and 2A (PP2A), which are potentially novel targets for anticancer therapies. Thus, cantharidin is exploited as anticancer drugs. Cantharidin is shown to be cytotoxic to cancer cells and stimulatory on the bone marrow. The renal toxicity of this drug has prevented its use in mainstream oncology.
Uses
Cantharidin (Cantharone), a mitochondrial poison derived from the blister beetle Cantharis vesicatoria, leads to changes in cell membranes, epidermal cell dyshesion, acantholysis, and blister formation and is also useful. Cantharidin can be compounded with salicylic acid and podophyllin resin; occlusion for only 2 hours is needed with this combination. Thick hyperkeratotic lesions should be pared down before painting. The lesion should then be painted with cantharidin, allowed to dry, and covered with Blenderm or other nonporous occlusive tape; 40% salicylic acid plaster may be used to achieve greater activity. The tape is left on for 24 hours or until the area begins to hurt. A blister, often hemorrhagic, will form, break, crust, and fall off in 7 to 14 days; at this time, the lesion is pared down, and any wart remnants are retreated. Because the effect of cantharidin is entirely intraepidermal, no scarring ensues. Ring-like or 'donut configuration' recurrences may be seen occasionally after treatment with cantharidin or, at times, following liquid nitrogen therapy. Owing to this agent’s toxicity, application by a physician is recommended. Verrusol, which contains 30% salicylic acid, 5% podophyllin, and 1% cantharidin, may be used in the same manner.
References
[1] Richard E. Honkanen (1993) Cantharidin, another natural toxin that inhibits the activity of serin/threonine protein phosphatases types 1 and 2A, 330, 283-286
[2] Jennette A. Sakoff, Stephen P. Ackland, Monique L. Baldwin, Mirella A. Keane, Adam
McCluskey (2002) Anticancer activity and protein phosphatase 1 and 2A inhibition of a new generation of cantharidin analogues, 20, 1-11
[3] Erin F. D. Mathes, Ilona J. Frieden (2010) Treatment of molluscum contagiosum with cantharidin: a practical approach, 39, 124-130
Description
Cantharidin (56-25-7) inhibits protein phosphatase 2A (Ki=0.19 μM) and PP1 (Ki=1.1 μM).1 Displays >500-fold selectivity over PP2B. Suppresses growth and migration of PANC-1 pancreatic cancer cells via phosphorylation and degradation of β-catenin.2?Cantharidin arrests G2/M transition via JNK/Sp1-dependent down-regulation of CDK1.3?Cell permeable. Warning: Avoid skin contact, may cause skin irritation or blistering.
Chemical Properties
white to light yellow crystal powde
Chemical Properties
Cantharidin is a brown to black powder.
Uses
Cantharidin is used as a potent inhibitor of PP2A phosphatase activity. It has been shown to stimulate cell cycle progression and induce premature mitosis, used topically (0.7%) as an anti-wart treatment, and has been shown to be active in cervical, tongue, neuroblastoma, bone, leukemia, ovarian, colon, and various other cancer cell lines.
Uses
Cantharidin is a natural toxin produced by blister beetles that moderately inhibits protein phosphatases 1 (PP1) (IC50 = 1.7 μM) and PP2A (IC50 = 0.2 μM) and only weakly inhibits the activity of PP2B (IC50 = 1 mM). It has been shown to stimulate cell cycle progression and induce premature mitosis, used topically (0.7%) as an anti-wart treatment, and has been shown to be active in cervical, tongue, neuroblastoma, bone, leukemia, ovarian, colon, and various other cancer cell lines.
Uses
In veterinary medicine as a vesicant, rubefa- cient, and counterirritant.
Definition
ChEBI: A monoterpenoid with an epoxy-bridged cyclic dicarboxylic anhydride structure secreted by many species of blister beetle, and most notably by the Spanish fly, Lytta vesicatoria. Natural toxin inhibitor of protein phosphatases 1 and 2A.
Indications
Cantharidin, from the beetle Cantharis vesicatoria, causes intraepidermal vesiculation
and is used in the treatment of warts and other benign cutaneous lesions.
Cantharidin is extremely toxic if taken internally; it should not be prescribed for
at-home use.
Cantharidin (Cantharone), a mitochondrial poison derived from the blister beetle Cantharis vesicatoria, leads to changes in cell membranes, epidermal cell dyshesion, acantholysis, and blister formation and is also useful. Cantharidin can be compounded with salicylic acid and podophyllin resin; occlusion for only 2 hours is needed with this combination. Thick hyperkeratotic lesions should be pared down before painting. The lesion should then be painted with cantharidin, allowed to dry, and covered with Blenderm or other nonporous occlusive tape; 40% salicylic acid plaster may be used to achieve greater activity. The tape is left on for 24 hours or until the area begins to hurt. A blister, often hemorrhagic, will form, break, crust, and fall off in 7 to 14 days; at this time, the lesion is pared down, and any wart remnants are retreated. Because the effect of cantharidin is entirely intraepidermal, no scarring ensues. Ring-like or "donut configuration" recurrences may be seen occasionally after treatment with cantharidin or, at times, following liquid nitrogen therapy. Owing to this agent’s toxicity, application by a physician is recommended. Verrusol, which contains 30% salicylic acid, 5% podophyllin, and 1% cantharidin, may be used in the same manner.
General Description
Brown to black powder or plates or scales. Formerly used as a counterirritant and vesicant. Used for the removal of warts. Used as an experimental anti tumor agent. Active ingredient in spanish fly, a reputed aphrodisiac.
Reactivity Profile
Organic anhydrides, such as Cantharidin, are incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Hazard
Extremely toxic; questionable carcinogen; powerful irritant to all cells and tissues; deadly poi- son; respiratory failure; death.
Health Hazard
Cantharidin is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg or a taste of less than 7 drops for a 70 kg (150 lb.) person. It is very toxic by absorption through skin.
Fire Hazard
When heated to decomposition Cantharidin emits acrid smoke and irritating fumes.
Biological Activity
Natural toxin inhibitor of protein phosphatases 1 and 2A (K i values are 1.1 and 0.19 μ M respectively); similar to okadaic acid (9,10-Deepithio-9,10-didehydroacanthifolicin ). Displays > 500-fold selectivity over PP2B.
Biochem/physiol Actions
Inhibitor of protein phosphatase 2A.
Potential Exposure
Formerly used as a counterirritant and vesicant. Also used for the removal of benign epithelial growths, e.g., warts. Used as an experimental antitumor agent. The active ingredient in “Spanish fly,” a reputed aphrodisia
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention. Foringestion, induce vomiting with syrup of ipecac.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
Cantharidin is not specifically listed in the DOT Performance-Oriented Packaging Standards with respect to labeling requirements or restrictions on shipping quantities.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged, and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
References
1) Honkanen (1993) Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A; FEBS Lett. 330 283 2) Wu et al. (2014) PP2A inhibitors suppress migration and growth of PANC-1 pancreatic cancer cells through inhibition on the Wnt/β-Catenin pathway by phosphorylation and degradation of β-catenin; Oncol. Rep., 32 513 3) Gong et al. (2015) PP2A inhibitors arrest G2/M transition through JNK/Sp1-dependent down-regulation of CDK1 and autophagy up-regulation of p21; Oncotarget, 6 18469
Cantharidin Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12814 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 1015 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29860 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860; +8618080483897 | sales@biopurify.com | China | 3772 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 1169 | 58 |
View Lastest Price from Cantharidin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-21 | Cantharidin
56-25-7
|
US $1.00 / kg | 1kg | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-21 | Cantharidin
56-25-7
|
US $0.00-0.00 / g | 1g | 98% | 2000 | Changsha Staherb Natural Ingredients Co., Ltd.
|
|
 |
2025-03-21 | Cantharidin
56-25-7
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd |
-

- Cantharidin
56-25-7
- US $1.00 / kg
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Cantharidin
56-25-7
- US $0.00-0.00 / g
- 98%
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- Cantharidin
56-25-7
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd