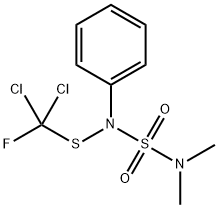
DICHLOFLUANID
- CAS No.
- 1085-98-9
- Chemical Name:
- DICHLOFLUANID
- Synonyms
- rman);Eparen;B-47531;ELVARON;Oiparen;Pecudin;EUPAREN;diparen;bay47531;Euparene
- CBNumber:
- CB0734712
- Molecular Formula:
- C9H11Cl2FN2O2S2
- Molecular Weight:
- 333.23
- MDL Number:
- MFCD00078639
- MOL File:
- 1085-98-9.mol
| Melting point | 110-112℃ (ethanol ) |
|---|---|
| Boiling point | 154°C (rough estimate) |
| Density | 1.5752 (rough estimate) |
| vapor pressure | 1.5 x 10-5 Pa (20 °C) |
| refractive index | 1.6000 (estimate) |
| Flash point | 2 °C |
| storage temp. | APPROX 4°C |
| Water Solubility | 1.3 mg l-1 (20 °C) |
| pka | -5.37±0.50(Predicted) |
| Merck | 13,3070 |
| BRN | 2947992 |
| CAS DataBase Reference | 1085-98-9 |
| EWG's Food Scores | 3 |
| FDA UNII | 76A92XU36Y |
| EPA Substance Registry System | Dichlofluanid (1085-98-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H319-H332-H400 |
| Precautionary statements | P261-P273-P280-P302+P352-P304+P340+P312-P305+P351+P338 |
| Hazard Codes | Xn,N,F |
| Risk Statements | 20-36-43-50/53-20/21/22-11-50 |
| Safety Statements | 24-37-60-61-36-26-16-36/37 |
| RIDADR | 2588 |
| WGK Germany | 3 |
| RTECS | WO6475000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Toxicity | LD50 in rats, guinea pigs, rabbits (mg/kg): 1.000 orally in all species (Grewe) |
DICHLOFLUANID price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | CRM04195 | Dichlofluanid certified reference material, TraceCERT? | 1085-98-9 | 100MG | $89.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 45433 | Dichlofluanid PESTANAL | 1085-98-9 | 250mg | $52.5 | 2024-03-01 | Buy |
| TRC | D434513 | Dichlofluanid | 1085-98-9 | 25mg | $80 | 2021-12-16 | Buy |
| Usbiological | 446661 | Dichlofluanid | 1085-98-9 | 25mg | $355 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | AGR0000168 | DICHLOFLUANID 98.00% | 1085-98-9 | 5G | $1155 | 2021-12-16 | Buy |
DICHLOFLUANID Chemical Properties,Uses,Production
Description
Dichlofluanid is solid sparingly soluble in water, soluble in most organic solvents, and decomposes in alkaline media.
Uses
Dichlofluanid is used to control a wide range of fungal diseases including storage diseases on many crops.
Uses
Fungicide.
Definition
ChEBI: A member of the class of sulfamides that is sulfamide in which the hydrogens attached to one of the nitrogens are replaced by methyl groups, while those attached to the other nitrogen are replaced by a phenyl and a [dichloro(fluoro)methyl]sulfanediyl group A fungicide introduced in 1965 and used in the cultivation of fruit and vegetables, as well as in wood preservatives, it is no longer approved for use in the European Union.
Metabolic pathway
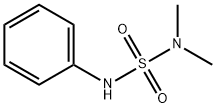
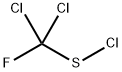
Dichlofluanid contains an unstable dichlorofluoromethylthio (sulfenyl) moiety that has been shown to undergo rapid hydrolytic and metabolic degradation to N',N'-dimethyl-N-phenylsulfamide (2) (dimethylsulfanilide). By analogy with captan, presumably the dichlorofluoromethylthio moiety can be transferred to the sulfur atoms of cellular thiols such as cysteine and glutathione. Thus in the presence of thiols, dichlofluanid is probably cleaved at the N-S bond to form thiophosgene (3) or its monofluoro analogue and other gaseous products such as hydrogen sulfide, hydrogen chloride and carbonyl sulfide. Thiophosgene or its monofluoro analogue is rapidly hydrolysed by water. The dichlorofluoromethylthio group and thiophosgene may be intermediates in the formation of addition products such as thiazolidine-2-thione-4-carboxylic acid (4) by addition to cysteine. A thiazolidine derivative of glutathione may also be formed (5).
Degradation
Dichlofluanid is hydrolysed rapidly in alkaline conditions to form N',N'-
dimethyl-N-phenylsulfamide (2). The hydrolytic DT50 is >15 days, >18
hours and <10 minutes at pH 4,7 and 9, respectively, at 22 °C (PM).
Dichlofluanid is unstable to light and its fungitoxicity decreases on
exposure, albeit to a lesser extent than for captan. Dichlofluanid does not
absorb light of wavelength of >295 nm and so photodegradation is
unlikely to occur in the absence of photosensitisers. Dichlofluanid was
reacted in vitru with glutathone or cysteine in water/methanol solutions.
The reaction was carried out in a closed system at 40 °C using traps for
COS and CO2 and analysis by TLC. The proposed route of degradation
is given in Scheme 1. Short-lived intermediates are proposed that were
not detected. It is not clear whether thiophosgene (3) or its monofluoro
analogue were formed (Schuphan ef al., 1981).
The photolysis of dichlofluanid was studied under artificial conditions
that may not be relevant to typical environmental circumstances.
Unlabelled dichlofluanid in methanol, benzene or acetone solution was
irradiated with a medium pressure UV lamp (100 W) but the emission
wavelengths were not given. As they photodegraded, the methanol
and benzene solutions gave a brown solid and the acetone solution
darkened. Products were separated and identified using IR, GC and MS
methods. The products from acetone solution were N',N'-dimethyl-Nphenylsulfamide
(2), phenyl isocyanate (6), phenyl isothiocyanate (7) and
dimethylamidosulfonyl chloride (8). Studies using GC-MS indicated the
presence of bis(dichlorofluoromethyl) disulfide (9). It was concluded that
irradiation of dichlofluanid produced mainly the hydrolysis product
dimethylsulfanilide (2) and the very active dichlorofluoromethyl sulfide radical (.SCCl2F), the latter reacting with other compounds in solution.
For example, 1-(dichlorofluoromethylthio)propan-2-one (10), and 1-
(dichlorofluoromethylsulfonyl)propan-2-one(11) were formed in acetone
solution by reaction with solvent. The phenyl isothiocyanate (12) was also
isolated In vitro tests against Botrytis cinerea showed that irradiation
decreased the fungicidal activity of dichlofluanid (Clark and Watkins,
1978).
DICHLOFLUANID Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | sales@senfeida.com | China | 23046 | 58 |
| Sichuan Kulinan Technology Co., Ltd | 400-1166-196 18981987031 | cdhxsj@163.com | China | 11707 | 57 |
| Spectrum Chemical Manufacturing Corp. | 021-021-021-67601398-809-809-809 15221380277 | marketing_china@spectrumchemical.com | China | 9658 | 60 |
| Chengdu Ai Keda Chemical Technology Co., Ltd. | 4008-755-333 18080918076 | 800078821@qq.com | China | 9718 | 55 |
| Shanghai T&W Pharmaceutical Co., Ltd. | +86 21 61551611 | China | 9891 | 58 | |
| Shanghai Yudiao Chemistry Technology Co.,Ltd | 21-37285211 18964703211 | info@yudiaochem.com | China | 255 | 58 |