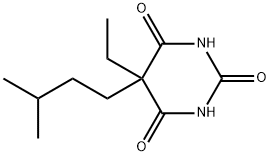
Amobarbital
- CAS No.
- 57-43-2
- Chemical Name:
- Amobarbital
- Synonyms
- Amal;Dorlo;Robarb;Somnal;Talamo;Amital;Amybal;Amytal;Isomyl;Mylodor
- CBNumber:
- CB0741976
- Molecular Formula:
- C11H18N2O3
- Molecular Weight:
- 226.27
- MDL Number:
- MFCD00057558
- MOL File:
- 57-43-2.mol
| Melting point | 156-158°C |
|---|---|
| Boiling point | 367.89°C (rough estimate) |
| Density | 1.1376 (rough estimate) |
| refractive index | 1.4620 (estimate) |
| Flash point | 9℃ |
| storage temp. | -20°C |
| pka | 8.0(at 25℃) |
| color | bitter crystals or leaflets from water |
| biological source | human blood |
| Water Solubility | <0.1 g/100 mL at 18.5 ºC |
| Stability | Stable. Incompatible with strong oxidizing agents. Hygroscopic. |
| CAS DataBase Reference | 57-43-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | GWH6IJ239E |
| ATC code | N05CA02 |
| NIST Chemistry Reference | Barbituric acid, 5-ethyl-5-isoamyl-(57-43-2) |
| EPA Substance Registry System | Amobarbital (57-43-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H336-H361d-H412 | |||||||||
| Precautionary statements | P202-P261-P264-P270-P273-P301+P310 | |||||||||
| Hazard Codes | F,T | |||||||||
| Risk Statements | 11-23/24/25-39/23/24/25 | |||||||||
| Safety Statements | 16-36/37-45 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 1 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2933530000 | |||||||||
| Hazardous Substances Data | 57-43-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in mice (mg/kg): 212 s.c. (Irrgang) | |||||||||
| DEA Controlled Substances | CSCN: 2126 CSA SCH: Schedule III NARC: No |
|||||||||
| NFPA 704 |
|
Amobarbital Chemical Properties,Uses,Production
Chemical Properties
Crystalline Solid
Originator
Hypnotal,Pharmacal
Uses
Controlled substance (depressant). Sedative, hypnotic
Uses
Amobarbital for binding at complex I to inhibit mitochondrial electron transport. Amobarbital is regulated as a schedule II compound in the United States and intended only for forensic and research purposes. This product is a qualified Reference Material (RM) that has been manufactured and tested to meet ISO17025 and Guide 34 guidelines. These materials are tested using validated analytical methods on qualified instrumentation to ensure traceability of measurements. All traceable RMs may be distinguished by their CofAs and can be downloaded below using the batch number located on the product label. For a representative CofA please contact our technical support.
Uses
Sedative, hypnotic. Controlled substance (depressant).
Definition
ChEBI: A member of the class of barbiturates that is pyrimidine-2,4,6(1H,3H,5H)-trione substituted by a 3-methylbutyl and an ethyl group at position 5. Amobarbital has been shown to exhibit sedative and hy notic properties.
Manufacturing Process
By interaction of malonic acid diethyl ester with sodium ethylate (molar ratio 1:1) and then with ethyl bromide (molar ratio 1:1) was prepared ethylmalonic acid diethyl ester. From ethylmalonic acid diethyl ester and sodium ethylate (molar ratio 1:1) and then with isopentylbromide was synthesized α-ethyl-α- isopentylmalonic acid diethyl ester. By condensation of α-ethyl-α- isopentylmalonic acid diethyl ester with urea in the presence of sodium ethylate was obtained ethyl-isopentylbarbituric acid.
brand name
Amytal Sodium (Lilly); Talamo (Marion Merrell Dow);Altinal;Alupent-sed;Ambese-la;Amobell;Amsal;Amycal;Amydorm;Amylbarb;Amylobeta;Analgilasa;Appenil;Asthmin;Beatol;Bludex;Calavon;Cuaot;Dexaspan;Dexital;Ergo-lonarid;Estimal;Etamyl;Ifenin;Isoamitil sedante;Isobec;Jalonac;Lonarid n;Medi-trol;Mudeka;Mylodorm sustrel;N 8;Neur-amyl;Novambobarb;Novogen;Obe_slim;Placidel;Protasma;Sedo-rythmodan;Sy-dexam;Transital.
Therapeutic Function
Hypnotic, Antiepileptic
World Health Organization (WHO)
Amobarbital is an intermediate-acting barbiturate which is controlled under Schedule III of the 1971 Convention on Psychotropic Substances. See WHO comment for barbiturates. (Reference: (UNCPS3) United Nations Convention on Psychotropic Substances (III), , , 1971)
General Description
White crystalline solid with no odor and a slightly bitter taste.
Air & Water Reactions
Amobarbital is hygroscopic . Insoluble in water.
Reactivity Profile
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Hazard
May be a habit forming drug of abuse.
Fire Hazard
Flash point data for Amobarbital are not available, however, Amobarbital is probably combustible.
Safety Profile
A poison by ingestion,intravenous, intraperitoneal, and subcutaneous routes. Seealso BARBITURATES. When heated to decomposition itemits toxic fumes of NOx.
Amobarbital Preparation Products And Raw materials
Raw materials
1of2