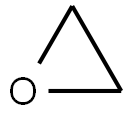
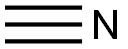3-Hydroxypropionitrile
- CAS No.
- 109-78-4
- Chemical Name:
- 3-Hydroxypropionitrile
- Synonyms
- 2-CYANOETHANOL;HYDRACRYLONITRILE;ETHYLENE CYANOHYDRIN;Hydroxypropanenitrile;3-HYDROXYPROPIONONITRILE;NSC 2598;usafrh-7;beta-Hpn;USAF rh-7;beta-Hpn3
- CBNumber:
- CB0852788
- Molecular Formula:
- C3H5NO
- Molecular Weight:
- 71.08
- MDL Number:
- MFCD00002826
- MOL File:
- 109-78-4.mol
- MSDS File:
- SDS
| Melting point | -46 °C (lit.) |
|---|---|
| Boiling point | 228 °C (lit.) |
| Density | 1.04 g/mL at 25 °C (lit.) |
| vapor density | 2.5 (vs air) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | diethyl ether: slightly soluble2.3% (w/w) at 15°C(lit.) |
| pka | 13.82±0.10(Predicted) |
| form | Liquid |
| Specific Gravity | 1.047 (20/4℃) |
| color | Clear slightly yellow to yellow |
| PH | 3.0-4.5 (H2O, 20℃)(undiluted) |
| explosive limit | 12.1% |
| Viscosity | 4.05mm2/s |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| Merck | 14,3794 |
| BRN | 635773 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents. Reacts with water or steam to release toxic vapours. Reacts violently with acids, amines, inorganic bases. Corrodes mild steel. |
| InChIKey | WSGYTJNNHPZFKR-UHFFFAOYSA-N |
| LogP | -0.94 at 20℃ |
| Indirect Additives used in Food Contact Substances | ETHYLENE CYANOHYDRIN |
| FDA 21 CFR | 175.105 |
| CAS DataBase Reference | 109-78-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 7V8108WN46 |
| NIST Chemistry Reference | Propanenitrile,3-hydroxy-(109-78-4) |
| EPA Substance Registry System | Ethylene cyanohydrin (109-78-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H373-H303-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a-P260-P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313-P314-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-24/25 | |||||||||
| RIDADR | 2810 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | MU5250000 | |||||||||
| F | 8-10 | |||||||||
| Autoignition Temperature | 922 °F | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29269095 | |||||||||
| Toxicity | LD50 orally in rats: 10.0 g/kg (Smyth, Carpenter) | |||||||||
| NFPA 704 |
|
3-Hydroxypropionitrile price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.18084 | 3-Hydroxypropionitrile for synthesis | 109-78-4 | 100mL | $41.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18084 | 3-Hydroxypropionitrile for synthesis | 109-78-4 | 500mL | $144 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18084 | 3-Hydroxypropionitrile for synthesis | 109-78-4 | 25kg | $2540 | 2024-03-01 | Buy |
| Sigma-Aldrich | 109924 | 3-Hydroxypropionitrile 97% | 109-78-4 | 250g | $71.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 109924 | 3-Hydroxypropionitrile 97% | 109-78-4 | 1kg | $155 | 2024-03-01 | Buy |
3-Hydroxypropionitrile Chemical Properties,Uses,Production
Chemical Properties
colourless or straw-coloured liquid
Chemical Properties
3-Hydroxypropionitrile is an aliphatic nitrile to which a hydroxyl group is attached at the ?-carbon. The nitrogen atom contains a lone pair of electrons, which is largely responsible for the polarization of the C≡N triple bond. Because of the greater electronegativity of nitrogen compared to carbon, nucleophilic compounds can attack the electrophilic carbon atom (Schaefer 1970), although the nitrogen atom is an excellent donor site for complexing with Lewis acids (Sheppard 1970). The hydroxyl functional group on the other end of the molecule is also polar; like the nitrile nitrogen, its oxygen is basic and nucleophilic. Reactions of the -OH group can involve the breaking of either of two bonds: the C-OH bond, with removal of the -OH group; or the O-H bond, with removal of -H. Either kind of reaction can involve substitution, in which a group replaces the -H or -OH, or elimination, in which a double bond is formed (Morrison and Boyd 1973). The aliphatic ethyl group separating the hydroxyl and nitrile groups, by its electron releasing inductive effect, lowers the reactivity of 3-Hydroxypropionitrile in nucleophilic addition reactions (Schaefer 1970) and stabilizes the compound. Apparently, when the hydroxyl group is in the beta position relative to the nitrile group, the compound is not readily hydrolyzed in the body to release cyanide (H?rtung 1982). When heated to decomposition, 3-Hydroxypropionitrile may produce toxic fumes of hydrogen cyanide, carbon monoxide, and nitrogen oxides (Lenga 1985). It will react with water or steam to produce toxic and flammable vapors (Sax 1984).
Occurrence
3-Hydroxypropionitrile does not occur as a natural product. Nevertheless, it may enter natural waters because it is present in concentrations between 2000 and 4000 p.p.m. in polyacrylimide polymers (Ikeda 1978) which are used as coagulants in water and wastewater purification. 3-Hydroxypropionitrile may leach from the polymer and enter the water being treated with the coagulant. Since acrylimide polymers are generally present at concentrations up to 1 p.p.m. during water purification (Sauerhoff et al 1976), if all the 3-Hydroxypropionitrile were extracted from the polymer, the concentration in water might be as high as 4 p.p.b. Fortunately, up to 99% of the 3-Hydroxypropionitrile may be removed by treatment with activated carbon in countercurrent multistage fluidized beds (Sasaoka 1975).
Uses
3-Hydroxypropionitrile has been used as polar solvent to investigate the orientation of the phospholipid at the surface of the polar solvents by neutral impact collision ion scattering spectroscopy and as a reagent for carboxyl protection in peptide synthesis. It has been used also in solid state composite electrolyte for dye-sensitized solar cells.
Uses
3-Hydroxypropionitrile has been used:
- as polar solvent to investigate the orientation of the phospholipid at the surface of the polar solvents by neutral impact collision ion scattering spectroscopy
- in solid state composite electrolyte for dye-sensitized solar cells
- as a reagent for carboxyl protection in peptide synthesis
Uses
Solvent for some cellulose esters and many inorganic salts.
Production Methods
3-Hydroxypropionitrile can be prepared by reacting ethylene chlorohydrin with sodium cyanide (Kendall and McKenzie 1923; Britton et al 1941; Fukui et al 1961), by reacting ethylene oxide and hydrogen cyanide in an alkaline medium (Badische Anilin and Soda-Fabrik 1966), or by treating an aqueous solution containing 2.5 vol% acrylonitrile with an alkali catalyst (Howsmon 1962).
General Description
Colorless to yellow-brown liquid with a weak odor. Sinks and mixes with water.
Air & Water Reactions
Water soluble. Reacts with water and steam to emit highly toxic, flammable vapors.
Reactivity Profile
3-Hydroxypropionitrile reacts violently with mineral acids, amines and inorganic bases. 3-Hydroxypropionitrile also reacts violently with chlorosulfonic acid, sulfuric acid, oleum and sodium hydroxide. 3-Hydroxypropionitrile is incompatible with bases, oxidizing agents, moisture and heat. 3-Hydroxypropionitrile is corrosive to mild steel.
Hazard
Toxic by ingestion.
Health Hazard
3-Hydroxypropionitrile is considered to be moderately hazardous by ingestion and skin contact, and slightly hazardous by inhalation (Parmeggiani 1983). Although there is no record of industrial poisoning from 3-Hydroxypropionitrile (Williams 1959), the liquid may cause eye irritation, and ingestion of the liquid may cause severe kidney damage (DeRenzo 1986). On the basis of available information, 3-Hydroxypropionitrile is not a carcinogen.
Flammability and Explosibility
Non flammable
Industrial uses
An 3-Hydroxypropionitrile feedstock was widely used for manufacturing acrylonitrile until an acetylene-based process began to replace it in 1953. Although the maximum use of 3-Hydroxypropionitrile to produce acrylonitrile occurred in 1963, acrylonitrile has not been produced by this route since 1970 (Cholod 1979). 3-Hydroxypropionitrile was also used in the first commercial process for manufacture of acrylic acid and acrylates. However, this route is no longer commercially significant (Cholod 1979). 3-Hydroxypropionitrile is a solvent for some cellulose esters and many inorganic salts (Stecher 1976). Basic dyes, as free base or inorganic or organic salt, are dissolved in 3-Hydroxypropionitrile to yield solutions especially useful for dyeing poly-acrylonitrile textiles (Farbenfabriken Bayer 1966). 3-Hydroxypropionitrile is added to nitrocellulose propellant compounds to provide a reasonably short cure cycle at room temperature (Lampert 1969). It is a selective washing solvent for the removal of carbon dioxide and other acidic gases from natural and process gas streams (Pure Oil Co. 1966). 3-Hydroxypropionitrile can also be used in the preparation of ?-alanine (Boatwright 1956), and has been used as a foundation fixative for road construction (Hirose et al 1980).
Metabolism
Organic cyanides, which include 3-Hydroxypropionitrile, are good examples of
compounds in which toxic action is related to mode of metabolism, since it
appears that toxicity, in many cases, is dependent on whether they can be
metabolized in the body to free cyanide ion, CNˉ (Williams 1959).
Sauerhoff et al (1976) studied the pharmacokinetics and metabolism of [14C]-
labelled 3-Hydroxypropionitrile in rats. 3-Hydroxypropionitrile labelled in the nitrile carbon was administered to male and female Sprague-Dawley rats at a dose level
of 20 mg/kg. The 3-Hydroxypropionitrile was absorbed from the gastrointestinal
tract, with peak plasma levels of [14C]-activity attained 4 h following administration
of the dose. Clearance of 14C from the plasma was biphasic, and 120 h after
the dose was administered, 86.7% of the total dose had been excreted. Of the 14C
excreted, 53.2% was eliminated in urine, 7.39% was excreted in feces, 0.44% was
expired as HCN, and 25.6% was expired as C02. Radioactivity in the urine was
attributed to three components: a conjugate of 3-Hydroxypropionitrile, ethylene
cyanohydrin, and thiocyanate. The net cyanide produced 48 h after administration
of 20 mg/kg 3-Hydroxypropionitrile was 80 μg. This level of cyanide production did
not appear to be toxicologically significant; as fast as it was formed, the free
cyanide was converted to thiocyanate and excreted. Cyanide was also eliminated
as HCN in expired air; virtually all the HCN had been eliminated 40 h after dosing.
The evidence provided by Sauerhoff et al (1976) indicated rapid elimination of
3-Hydroxypropionitrile from the body and a low rate of conversion to CNˉ
Korshunov (1970) was unable to detect any cyanide in the blood of rats 1 to 36
hours after administration of 3 g/kg 3-Hydroxypropionitrile.
To examine the oral toxicity of nitriles without the effect of cyanide, Tanii and
Hashimoto (1984a, 1984b) pretreated mice with CC14, a chemical known to impair
the microsomal monooxygenase system and prevent the the release of cyanide
from nitriles. Carbon tetrachloride pretreatment greatly reduced the mortality
produced by 3-Hydroxypropionitrile in mice, thus implicating cyanide in the acute
toxicity of 3-Hydroxypropionitrile.
Tanii and Hashimoto (1986) also studied the effect of ethanol on the metabolism
of 20 nitriles, including 3-Hydroxypropionitrile. When mice were dosed orally with
either ethanol (4.0 g/kg) or glucose (7.0 g/kg), the hepatic metabolizing activity of
nitriles, and hence the amount of cyanide released, for the ethanol-treated group
was always higher than that for the glucose-treated control group, although no
change in the content of hepatic microsomal P-450 was observed between the two
groups. On the other hand, ethanol inhibited the in vitro metabolism of most of the
nitriles examined. However, since the metabolism of nitriles was stimulated 13 h
after ethanol dosing, the stimulating effect may have overwhelmed its initial
inhibitory action. This study suggested that ethanol could enhance the acute
toxicity of nitriles such as 3-Hydroxypropionitrile.
Sauerhoff et al (1976) did not identify the conjugate of 3-Hydroxypropionitrile
that was excreted in the urine of rats dosed with 3-Hydroxypropionitrile. Cyanoacetic
acid, however, was detected in the urine of rats following administration of
3-Hydroxypropionitrile (Lipton et al 1958). Merkow et al (1959) also observed that
3-Hydroxypropionitrile was detoxified by conversion to cyanoacetic acid in vivo.
3-Hydroxypropionitrile was oxidized to an aldehyde by rat liver preparations
containing an active alcohol oxidase (Bernheim and Handler 1941).
3-Hydroxypropionitrile Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TAIZHOU DKD CHEMICAL CO., LTD. | +8618658336633 | yf.chen@tzdkdchem.com | China | 134 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | niki@zlchemi.com | China | 1149 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1803 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 | info@royal-chem.com | China | 114 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
View Lastest Price from 3-Hydroxypropionitrile manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | 3-Hydroxypropionitrile
109-78-4
|
US $5.00 / kg | 1kg | ≥98.5% | 500mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-11-28 | 3-Hydroxypropionitrile
109-78-4
|
US $10.00 / kg | 1kg | 99% | 20 ton | Hebei Zhuanglai Chemical Trading Co Ltd | |
 |
2024-10-25 | 3-Hydroxypropionitrile
109-78-4
|
US $0.00 / kg | 25kg | 98% | 20MT/Month | Hebei Yanxi Chemical Co., Ltd. |
-

- 3-Hydroxypropionitrile
109-78-4
- US $5.00 / kg
- ≥98.5%
- Jinan Finer Chemical Co., Ltd
-

- 3-Hydroxypropionitrile
109-78-4
- US $10.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co Ltd
-

- 3-Hydroxypropionitrile
109-78-4
- US $0.00 / kg
- 98%
- Hebei Yanxi Chemical Co., Ltd.