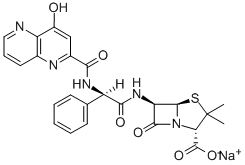
apalcillin sodium
- CAS No.
- 58795-03-2
- Chemical Name:
- apalcillin sodium
- Synonyms
- APALCILLIN SODIUM;6α-[[(R)-[[(4-Hydroxy-1,5-naphthyridin-3-yl)carbonyl]amino]phenylacetyl]amino]penicillanic acid sodium salt;(2S,5R,6R)-6-[[(R)-(4-Hydroxypyrido[3,2-b]pyridin-3-ylcarbonylamino)phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid sodium salt;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[[(4-hydroxy-1,5-naphthyridin-3-yl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, monosodium salt, [2S-[2alpha,5alpha,6beta(S*)]]-
- CBNumber:
- CB0892957
- Molecular Formula:
- C25H22N5NaO6S
- Molecular Weight:
- 543.52685
- MDL Number:
- MOL File:
- 58795-03-2.mol
| FDA UNII | 3XQ12NYC0Q |
|---|
apalcillin sodium Chemical Properties,Uses,Production
Originator
Lumota,Thomae,W. Germany,1982
Uses
Antibacterial.
Definition
ChEBI: Apalcillin sodium is an organic sodium salt. It contains an apalcillin(1-).
Manufacturing Process
(a) Preparation of 6-D-α-aminobenzylpenicillin phenacyl ester: To a
suspension of phenacyl 6-aminopenicillanate hydrochloride (1.85 g) and Dphenylglycyl
chloride hydrochloride (1.29 g) in dichloromethane (20 ml),
sodium bicarbonate (1.05 g) was added, and the resultant mixture was stirred while cooling with ice for 6 hours. The reaction mixture was filtered to
eliminate the by-produced sodium chloride. The filtrate was admixed with
isopropanol and concentrated under reduced pressure by the aid of a rotary
evaporator. After the evaporation of dichloromethane, the precipitate was
collected by filtration to give the objective compound in the form of the
hydrochloride (2.19 g) MP 142° to 148°C (decomposition).
(b) Preparation of D-α-(4-hydroxy-1,5-naphthyridine-3-
carbonamido)benzylpenicillin: To a solution of 6-D-α-aminobenzylpenicillin
phenacyl ester (hydrochloride) (2.01 g) and triethylamine (0.808 g) in
dimethylformamide (20 ml), 4-hydroxy-1,5-naphthyridine-3-carboxylic acid Nsuccinimide
ester [MP 310° to 311°C (decomposition)] (1.15 g) was added
while cooling with ice, and the resultant mixture was stirred for 1 hour.
Stirring was further continued at room temperature for 2 hours. After cooling
with ice, 1% sodium bicarbonate solution (100 ml) was added thereto. The
precipitated crystals were collected by filtration, washed with water and dried
over phosphorus pentoxide to give D-(α-4-hydroxy-1,5-naphthyridine-3-
carboxamido)benzylpenicillin phenacyl ester (2.17 g).
The above product was dissolved in dimethylformamide (65 ml), sodium
thiophenoxide (0.89 g) was added thereto, and the resultant mixture was
stirred at room temperature for 1 hour. To the resultant mixture, acetone (650
ml) was added, and the separated crystals were collected by filtration and
washed with acetone and ether in order to give the objective compound in the
form of the sodium salt (1.3 g).
In the above procedure, the use of 4-hydroxy-1,5-naphthyridine-3-carbonyl
chloride in place of 4-hydroxy-1,5-naphthyridine-3-carboxylic acid Nsuccinimide
ester can also afford the same objective compound as above. The
use of sodium thio-n-propoxide in place of sodium thiophenoxide can also give
the objective compound in the form of the sodium salt.
Therapeutic Function
Antibacterial
apalcillin sodium Preparation Products And Raw materials
Raw materials
Preparation Products
apalcillin sodium Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49734 | 58 |
| Supplier | Advantage |
|---|---|
| Hangzhou MolCore BioPharmatech Co.,Ltd. | 58 |