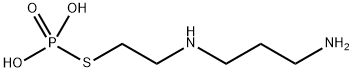
Amifostine
- CAS No.
- 20537-88-6
- Chemical Name:
- Amifostine
- Synonyms
- Ethyol;γphos;sapep;Ethyo;WR2721;apaetp;CS-271;wr2721c;Ethiotas;ym-08310
- CBNumber:
- CB1316377
- Molecular Formula:
- C5H15N2O3PS
- Molecular Weight:
- 214.22
- MDL Number:
- MFCD00233058
- MOL File:
- 20537-88-6.mol
- MSDS File:
- SDS
| Melting point | 160-1610C |
|---|---|
| Boiling point | 441.7±51.0 °C(Predicted) |
| Density | 1.367±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in Water. |
| form | powder |
| pka | 1.28±0.10(Predicted) |
| color | White |
| Water Solubility | Soluble to 100 mM in water |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 20537-88-6(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | amifostine; Ethyol |
| FDA UNII | ILA426L95O |
| NCI Drug Dictionary | amifostine |
| ATC code | V03AF05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | TE6491000 |
| Toxicity | cyt-mus-ipr 300 mg/kg CUSCAM 54,1080,85 |
Amifostine price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Tocris | 6655 | Amifostine | 20537-88-6 | 100 | $102 | 2021-12-16 | Buy |
| TRC | A576810 | Amifostine | 20537-88-6 | 100mg | $55 | 2021-12-16 | Buy |
| ChemScene | CS-2875 | Amifostine ≥98.0% | 20537-88-6 | 10mg | $65 | 2021-12-16 | Buy |
| AK Scientific | H037 | Amifostine | 20537-88-6 | 5mg | $34 | 2021-12-16 | Buy |
| ChemScene | CS-2875 | Amifostine ≥98.0% | 20537-88-6 | 50mg | $272 | 2021-12-16 | Buy |
Amifostine Chemical Properties,Uses,Production
Description
Amifostine, an organic thiophosphate, was introduced for the reduction of cisplatin-induced renal toxicity in patients with advanced ovarian cancer. It is also a radio-protective agent. Amifostine is a prodrug which is rapidly dephosphorylated, preferentially in non-tumor tissues to the active thiol. This agent binds to chemotherapeutic drugs and free radicals released by radiotherapy. The protective effect of amifostine was observed in a wide range of normal organs including bone marrow and gastrointestinal mucosa without interfering with the anti-tumor effect of chemohadiotherapy, indicating an increased therapeutic index for existing cancer treatment. Amifostine is also being evaluated as a cytoprotective in other types of tumors including lung, breast, head and neck cancers. It was reported to be a potent mucolytic with potential in cystic fibrosis.
Chemical Properties
White Solid
Originator
US Bloscience (U.S.A.)
Uses
neuroleptic
Uses
It is a thiophosphate derivative of cysteamine; provides normal cells with selective protection against the toxic effects of cancer chemotherapy and radiation treatment
Definition
ChEBI: An organic thiophosphate that is the S-phospho derivative of 2-[(3-aminopropyl)amino]ethanethiol. A prodrug for the free thiol, WR-1065, which is used as a cytoprotectant in cancer chemotherapy and radiotherapy.
Manufacturing Process
A solution of 2-(3-aminopropylamino)ethanol (25.0 g, 0.212 mole) in 48 % hydrobromic acid (200 ml) was distilled until 35 ml of distillate had been collected. The solution was refluxed and, periodically, more distillate was collected. The total volume of distillate removed in 7 distillation periods was 160 ml, or 80 % of the original volume of 48% hydrobromic acid, and the time of continuous boiling was approximately 48 hours. The residual solution was then evaporated to dryness under reduced pressure with the aid of several added portions of methanol. The crystalline residue was thoroughly triturated with acetone, collected, and washed on the funnel with acetone. After the product had been pressed as dry as possible on the funnel, it was dissolved in a slight excess of boiling methanol and the solution was filtered. Addition of acetone to the filtrate precipitated pure N-(2-bromoethyl)-1,3- propanediamine dihydrobromide as colorless crystals, which were dried in vacuum over phosphorus pentoxide: yield 58.0 g (80%), melting point 205- 206°C. Trisodium phosphorothioate (6.93 g, 38.5 mmoles) was gradually added in small portions with vigorous stirring to water (38 ml) cooled externally by means of a water bath (15°-20°C). To the resulting suspension was added N-(2-bromoethyl)-1,3-propanediamine dihydrobromide (13.3 g, 38.8 mmoles). After a few minutes, complete solution occurred, and N,Ndimethylformamide (19 ml) was added with continued external cooling at 15°- 20°C. After the solution had been stirred at about 20°C for 90 min, it was poured into methanol (250 ml), and the mixture was refrigerated at 4°C overnight. The white precipitate that formed was collected and pressed as dry as possible on the funnel. The solid was dissolved in water (40 ml), and the solution was filtered. Addition of methanol (250 ml) reprecipitated the product. After the mixture had been refrigerated about 1 hour, the product was collected and washed on the funnel, first with methanol and finally with ether. The white solid was dried in vacuo at room temperature, then exposed to ambient conditions of the laboratory for 5 hours, and bottled under nitrogen and stored in a freezer. The yield of S-2-(3-aminopropylamino)ethyl dihydrogen phosphorothioate monohydrate, melting point (from methanol) 160-161°C, dec., was 8.15 g (91%).
brand name
Ethyol (MedImmune) [USAN previously used: Ethiofos.].
Therapeutic Function
Radioprotective, Chemoprotectant, Mucolytic
Safety Profile
Poison by intravenous,intramuscular, and intraperitoneal routes. Moderately toxicby ingestion. An experimental teratogen. Otherexperimental reproductive effects. Mutation data reported. When heated todecomposition it emits very toxic fumes of SOx, PO
storage
Store at +4°C
References
1) Capizzi et al. (2000), Chemoprotective and radioprotective effects of amifostine: an update of clinical trials; Int. J. Hematol., 72 425 2) Provinciali et al. (1999), In vivo amifostine (WR-2721) prevents chemotherapy-induced apoptosis of peripheral blood lymphocytes from cancer patients; Life Sci., 64 1525 3) Koukourakis et al. (2004), Amifostine before chemotherapy: improved tolerance profile of the subcutaneous over the intravenous route; Cancer Chemother. Pharmacol., 53 8 4) Maurici et al. (2001), Amifostine (WR2721) restores transcriptional activity of specific p53 mutant proteins in a yeast functional assay; Oncogene, 20 3533
Amifostine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | info@longyupharma.com | China | 2538 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Hebei Bonster Technology Co.,Limited | +8613315996897 | bsterltd.wendy@gmail.com | China | 792 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34553 | 58 |
View Lastest Price from Amifostine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Amifostine
20537-88-6
|
US $0.00 / g | 1g | 98.0% | 5kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-16 | Amifostine
20537-88-6
|
US $31.00-108.00 / mg | 99.88% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-10-30 | Amifostine
20537-88-6
|
US $10.00 / Kg/Bag | 1Kg/Bag | 99% | 20 Tons | Hebei Zhanyao Biotechnology Co. Ltd |
-

- Amifostine
20537-88-6
- US $0.00 / g
- 98.0%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Amifostine
20537-88-6
- US $31.00-108.00 / mg
- 99.88%
- TargetMol Chemicals Inc.
-

- Amifostine
20537-88-6
- US $10.00 / Kg/Bag
- 99%
- Hebei Zhanyao Biotechnology Co. Ltd
20537-88-6(Amifostine)Related Search:
1of4