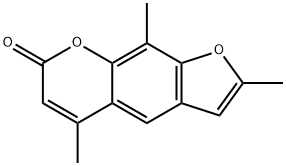
TRIOXSALEN
- CAS No.
- 3902-71-4
- Chemical Name:
- TRIOXSALEN
- Synonyms
- LACTONE;NSC-71047;Trioxalen;Trioxsale;Trixsalen;rioxsalen;TRIOXSALEN;TRISORALEN;Elder 8011;TRIOXSALIN
- CBNumber:
- CB1363250
- Molecular Formula:
- C14H12O3
- Molecular Weight:
- 228.24
- MDL Number:
- MFCD00005010
- MOL File:
- 3902-71-4.mol
- MSDS File:
- SDS
| Melting point | 229-231 °C(lit.) |
|---|---|
| Boiling point | 310.05°C (rough estimate) |
| Density | 1.2196 (rough estimate) |
| refractive index | 1.5557 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble |
| form | powder |
| color | white |
| Merck | 14,9735 |
| BRN | 221723 |
| CAS DataBase Reference | 3902-71-4 |
| FDA UNII | Y6UY8OV51T |
| ATC code | D05AD01,D05BA01 |
| IARC | 3 (Vol. 40, Sup 7) 1987 |
| EPA Substance Registry System | 7H-Furo[3,2-g][1]benzopyran-7-one, 2,5,9-trimethyl- (3902-71-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H312+H332-H314-H341 | |||||||||
| Precautionary statements | P202-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34-40 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 1759 8/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | LV1576000 | |||||||||
| F | 8-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29322985 | |||||||||
| NFPA 704 |
|
TRIOXSALEN price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T6137 | Trioxsalen ≥98% (HPLC), powder | 3902-71-4 | 1g | $481 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR3257 | Trioxsalen pharmaceutical secondary standard, certified reference material | 3902-71-4 | 500MG | $230 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHL80322 | Trioxsalen phyproof? Reference Substance | 3902-71-4 | 50mg | $262 | 2024-03-01 | Buy |
| TCI Chemical | T2267 | Trioxsalen >98.0%(HPLC) | 3902-71-4 | 1g | $37 | 2024-03-01 | Buy |
| TCI Chemical | T2267 | Trioxsalen >98.0%(HPLC) | 3902-71-4 | 5g | $107 | 2024-03-01 | Buy |
TRIOXSALEN Chemical Properties,Uses,Production
Chemical Properties
White to slightly beige crystalline powder
Originator
Trisoralen,Elder,US,1965
Uses
melanizing agent, antipsoriatic
Uses
Trioxsalen has been used:
- to induce small deletion mutations in worms
- in combination with ultraviolet A (UVA)
- to induce interstrand crosslinks (ICLs) in DNA
- for the preparation and photoactivation of trimethyl psoralen
Indications
Trioxsalen (Trisoralen) followed by UVA exposure is used to repigment vitiliginous areas and in photochemotherapy.
Definition
ChEBI: 7H-Furo[3,2-g]chromen-7-one in which positions 2, 5, and 9 are substituted by methyl groups. Like other psoralens, trioxsalen causes photosensitization of the skin. It is administered orally in conjunction with UV-A for phot therapy treatment of vitiligo. After photoactivation it creates interstrand cross-links in DNA, inhibiting DNA synthesis and cell division, and can lead to cell injury; recovery from the cell injury may be followed by increased melanisation of the epidermi .
Manufacturing Process
(A) Preparation of 7-Hydroxy-4,8-Dimethylcoumarin: Chilled ethyl
acetoacetate (157 ml, 1.20 mols) followed by 2-methyl-resorcinol (130 g,
1.04 mols) was dissolved in well-stirred concentrated sulfuric acid (600 ml) at
such a rate as to keep the temperature below 10°C (ice bath). The stirred
solution was allowed to warm gradually and after 3 hours was added to water
(ca 8 liters) with mechanical stirring. The product was collected, washed twice
with water, and dried at 70° to 80°C until the first sign of darkening. Yield
191.3 g (95.4%). Recrystallization from aqueous ethanol gave 7-hydroxy-4,8-
dimethylcoumarin as colorless needles, MP 260.5° to 261°C. In dilute sodium
hydroxide, the compound gives a yellow solution which exhibits blue
fluorescence. (B) Preparation of 7-Allyloxy-4,8-Dimethylcoumarin: 7-Hydroxy-4,8-
dimethylcoumarin (191.3 g, 1.01 mols), anhydrous potassium carbonate (604
g, 4.37 mols), and allyl bromide (578 ml, 6.22 mols) were refluxed overnight
in acetone (ca 3 liters) with mechanical stirring. The reaction mixture was
concentrated nearly to dryness on a steam bath under reduced pressure,
water (ca 8 liters) was added, and the product was collected by filtration. It
was washed with 5% NaOH and water (ca 1.5-liter portions) and was dried in
a vacuum desiccator. The dry solid was washed with petroleum ether (30° to
60°C) to remove excess allyl bromide. Removal of the petroleum ether under
reduced pressure left 210.0 g (90.7% yield) of product. The 7-allyloxy-4,8-
dimethylcoumarin was crystallized from aqueous ethanol as colorless needles,
MP 108° to 109°C.
(C) Preparation of 6-Allyl-7-Hydroxy-4,8-Dimethylcoumarin: 7-Allyloxy-4,8-
dimethylcoumarin (195.0 g, 0.84 mol) was heated (oil bath) to 2154°C
(reaction mixture temperature) for 3 hours and was then poured into absolute
alcohol (ca 1.5 liters). Activated carbon (Norite) (19.5 g) was added, and the
solution was heated to boiling, filtered, and diluted with excess water (ca 12
liters). The product was collected by filtration and partially dried at 70°C for 6
hours. 6-Allyl-7-hydroxy-4,8-dimethylcoumarin was obtained as pale yellow
microcrystalline prisms, MP 166° to 168°C, by two recrystallizations from
aqueous ethanol of a portion of the partially dried solid. The remaining
partially dried solid was used in the next step.
(D) Preparation of 7-Acetoxy-6-Allyl-4,8-Dimethylcoumarin: A solution of the
partially dried 6-allyl-7-hydroxy-4,8-dimethylcoumarin obtained in the
previous step, acetic anhydride (915 ml, 9.7 mols) and fused sodium acetate
(2 g) was refluxed for 4 hours and added to water (ca 8 liters) with mechanical stirring. After excess acetic anhydride had decomposed, the 7-
acetoxy-6-allyl-4,8-dimethylcoumarin was collected by filtration, dried, and
recrystallized from absolute alcohol, MP 144.5° to 145.5°C. Yield 145.4 g
(63.8%, based on 7-allyloxy-4,8-dimethylcoumarin).
(E) Preparation of 7-Acetoxy-6-(2',3'-Dibromopropyl)-4,8-Dimethylcoumarin:
7-Acetoxy-6-allyl-4,8-dimethylcoumarin (145.4 g, 0.534 mol) was dissolved in
chloroform (ca 800 ml). The stirred solution was cooled in an ice bath and
bromine (85.2 g, 0.534 mol) in chloroform (200 ml) was added at such a rate
as to keep the temperature below 25°C. Evaporation of chloroform on the
steam bath left an off-white residue of the crude dibromide. Yield 230.6 g
(quantitative). 7-Acetoxy-6-(2',3'-dibromopropyl)-4,8-dimethylcoumarin was
crystallized from ethanol as colorless prisms, MP 141.5° to 142.5°C.
(F) Preparation of 2',4,8-Trimethylpsoralen: Crude 7-acetoxy-6-(2',3'-
dibromopropyl)-4,8-dimethylcoumarin (245.7 g, 0.57 mol) was refluxed for 1
1/2 hours with a stirred solution of sodium (65.4 g, 2.85 mols) in a
magnesium-dried ethanol (2.1 liters). After standing at room temperature for
15 minutes, the reaction mixture was poured into a stirred mixture of ice
(8,000 g) and a 3.5% HCl (8 liters). Twelve hours later, the precipitate had
coagulated and was collected by filtration; it was thoroughly washed with
successive 3-liter portions of 5% NaOH, water, 0.5% HCl, and water.
After partial drying at 60°C for 5 hours, the crude trimethylpsoralen material
was thoroughly dried in a vacuum desiccator. Yield 110.1 g (85%). Fractional
crystallization, using activated carbon (Norite) (30.8 g), from mixtures of
chloroform and petroleum ether (30° to 60°C) and finally from chloroform
alone gave colorless prisms of 2',4,8-trimethylpsoralen, MP 234.5° to 235°C.
Yield 61.8 g (48%)
brand name
Trisoralen (Valeant).
Therapeutic Function
Dermal pigmentation enhancer
Biological Activity
trioxsalen has been reported to intercalate into dna forming dna single-strand adducts and interstrand crosslinks when activated with uv light.
Biochem/physiol Actions
Photochemical crosslinker of DNA that has been used as a probe for nucleic acid structure and function. Trioxsalen has also been used to crosslink DNA onto mica surfaces.
in vitro
trioxsalen (trimethylpsoralen, trioxysalen or trisoralen) is a furanocoumarin and a psoralen derivative. it is obtained from several plants, mainly psoralea corylifolia. like other psoralens it causes photosensitization of the skin. after photoactivation it creates interstrand cross-links in dna, which can cause programmed cell death unless repaired by cellular mechanisms. in research it can be conjugated to dyes for confocal microscopy and used to visualize sites of dna damage [1].
in vivo
mice received 3h-trioxsalen either orally or intraperitoneally. it was found that over 88% of trioxsalen, after po or i.p. administration, was excreted in the urine within 8 hours and over 90% within 12 hours. the distribution patterns of trioxsalen radioactivity demonstrated that trioxsalen was selectively present in liver, skin, and blood and was barely detectable in other organs. the highest values were observed between 2 and 6 hours and diminished rapidly thereafter [1].
Purification Methods
Purify trioxsalen by recrystallisation from CHCl3. If too impure, it is fractionally crystallised from CHCl3/pet ether (b 30-60o) using Norit and finally crystallised from CHCl3 alone to give colourless prisms, m 234.5-235o. It is a photosensitiser so it should be stored in the dark. [UV: Kaufmann J Org Chem 26 117 1961, Baeme et al. J Chem Soc 2976 1949, Beilstein 19/4 V 472.]
References
[1] mahmoud m. a. hassan. trioxsalen. analytical profiles of drug substances volume 10, 1981, pages 705-727.
TRIOXSALEN Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9639 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 | |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | sales@senfeida.com | China | 23046 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52924 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
3902-71-4(TRIOXSALEN)Related Search:
1of4