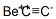Beryllium carbide
- CAS No.
- 506-66-1
- Chemical Name:
- Beryllium carbide
- Synonyms
- Einecs 208-050-7;beryllium carbide;beryllium acetylide;Beryllium dicarbide;1,3-Diberyllapropadiene;Beryllium carbide (be2C);Methanetetrayldiberyllium
- CBNumber:
- CB1920170
- Molecular Formula:
- C2Be
- Molecular Weight:
- 33.033582
- MDL Number:
- MOL File:
- 506-66-1.mol
| Melting point | decomposes at >2100℃ [MER06] |
|---|---|
| Density | 1.90 |
| solubility | reacts with H2O |
| form | red cubic crystals |
| color | red cubic crystals, crystalline |
| Water Solubility | decomposed very slowly by H2O; hydrolysis yields methane and beryllium hydroxide [KIR78] [MER06] |
| Crystal Structure | Cubic |
| CAS DataBase Reference | 506-66-1 |
| FDA UNII | F5D2F26ONX |
| EPA Substance Registry System | Beryllium carbide (Be2C) (506-66-1) |
Beryllium carbide Chemical Properties,Uses,Production
Chemical Properties
Red cubic crystal; hard and refractory; density 1.90 g/cm3; decomposes when heated above 2,100°C; reacts with water.
Physical properties
Brick-red or yellowish-red octahedra. Uses: Nuclear reactor cores.
Uses
Beryllium acetylide is used in a nuclear reactor as core material,Nuclear reactor core material: Schwartz.
Uses
Nuclear reactor core material: Schwartz, US 3170812 (1965 to USAEC).
Uses
Beryllium carbide (Be2C) is used for the cores in nuclear reactors.
Preparation
Beryllium carbide is prepared by heating the elements beryllium and carbon at elevated temperatures (above 900°C). It also may be prepared by reduction of beryllium oxide with carbon at a temperature above 1,500°C:
2BeO + 3C → (1500℃)→ Be2C + 2CO
Beryllium carbide decomposes very slowly in water:
Be2C + 2H2O → 2BeO + CH4
The rate of decomposition is faster in mineral acids with evolution of methane. However, in hot concentrated alkalies the reaction is very rapid, forming alkali metal beryllate and methane:
Be2C + 4NaOH → 2Na2BeO2 + CH4
Beryllium carbide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |
Related articles
- What is the crystal structure of Beryllium carbide?
- Beryllium carbide in its pure form is a colorless transparent crystal historically identified by Lebeau with Be2C composition.....
- Apr 1,2024