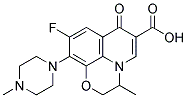
OFLOXACIN
- CAS No.
- 83380-47-6
- Chemical Name:
- OFLOXACIN
- Synonyms
- TARIVID;KFLOXACIN;AKOS NCG1-0050;Mesylate ofloxacin
- CBNumber:
- CB2103600
- Molecular Formula:
- C18H20FN3O4
- Molecular Weight:
- 361.37
- MDL Number:
- MFCD00226105
- MOL File:
- 83380-47-6.mol
- MSDS File:
- SDS
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H332-H312-H341-H334-H317-H302 |
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312-P201-P202-P281-P308+P313-P405-P501-P280-P302+P352-P312-P322-P363-P501 |
| Safety Statements | 22-24/25 |
| WGK Germany | 2 |
| RTECS | UU8815500 |
OFLOXACIN Chemical Properties,Uses,Production
Originator
Exocin,Allergan
Definition
ChEBI: Ofloxacin is a racemate comprising equimolar amounts of levofloxacin and dextrofloxacin. It is a synthetic fluoroquinolone antibacterial agent which inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication. It has a role as a DNA synthesis inhibitor, an antiinfective agent, an antibacterial drug, an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor and a topoisomerase IV inhibitor. It is a quinolone antibiotic, a fluoroquinolone antibiotic and a racemate. It contains a levofloxacin and a dextrofloxacin.
Manufacturing Process
20 g of 2,3,4-trifluoronitrobenzene was dissolved in 150 ml of dimethyl sulfoxide, and to this mixture a solution of 10% potassium hydroxide was added dropwise while keeping the temperature at 18° to 20°C. Then, the mixture was stirred for 2 hours at room temperature and one liter of water was added to this reaction mixture and the mixture was shaken with chloroform. The water layer was acidified with hydrochloric acid and was extracted with chloroform. The extract was washed with water and was dried, then chloroform layer was concentrated. The residue was purified by silica gel column chromatography to provide 5.8 g of 2,3-difluoro-6-nitrophenol as yellow oil.
7.9 g of the 2,3-difluoro-6-nitrophenol, 50.1 g of 1,2-dibromoethane and 18.7 g of potassium carbonate were added to 80 ml of dimethylformamide and the mixture was stirred for 2.5 hours at from about 80° to 100°C (bath temperature). The reaction mixture was concentrated to dryness in vacuo and the residue was distributed between ethyl acetate and water. The organic solvent layer was washed with water and was dried, then the solvent was evaporated. The residue was dissolved in benzene and was purified by silica gel column chromatography to provide 7.7 g of 2-(2-bromoethoxy)-3,4difluoronitrobenzene as light yellow oil.
1.74 g of this product was dissolved in 30 ml of methanol and a solution of 6.44 g of sodium dithionite dissolved in 15 ml of water was added thereto. The mixture was stirred for 1 hour at room temperature. Methanol was evaporated and the residue was extracted with chloroform. After the extract was washed with water and dried, the solvent was evaporated to provide 0.44 g of 2-(2-bromoethoxy)-3,4-difluoroaniline.
1.82 g of this product and 3.03 g of potassium carbonate were added to 10 ml of dimethylformamide and the mixture was stirred for 1 hour at from about 80° to 100°C (bath temperature). The reaction mixture was added to ice-cold water and was extracted with ethyl acetate. After the extract was washed with water and dried, the solvent was distilled off at room temperature to provide 1.21 g of 7,8-difluoro-2,3-dihydro-4H-[1,4]benzoxazine with m.p. 48°-54°C.
The mixture of 1.1 g of this product and 1.38 g of diethyl
ethoxymethylenemalonate was stirred for 2 hours at from about 130° to 135°C (bath temperature). The ethanol produced was evaporated and 20 g of ethyl polyphosphate was added to the residue. Then the mixture was stirred for 1.5 hours at from about 140° to 145°C (bath temperature). The reaction mixture was added to ice-cold water and was extracted with chloroform. The extract was washed fully with water. After drying, the solvent was evaporated and the residue was recrystallized from ethyl acetate. 1.3 g of ethyl 9,10difluoro-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6carboxylate was obtained as colorless needles with m.p. 265°-266°C.
1.15 g of this product was added to 12 ml of mixture of concentrated hydrochloric acid and acetic acid (1:4 by volume) and the mixture was stirred for 4 hours at 100° to 110°C (bath temperature). After cooling, the precipitated crystals were collected by filtration, washed with water, methanol and chloroform to give 0.78 g of 9,10-difluoro-7-oxo-2,3-dihydro-7Hpyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid as colorless needles with m.p. above 300°C.
1.0 g of 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3de][1,4]benzoxazine-6-carboxylic acid and 2.85 g of N-methylpiperazine were added to 15 ml of dimethylsulfoxide. The mixture was stirred at a temperature of from about 100° to 110°C (bath temperature) for 12 hours and the reaction mixture was concentrated to dryness in vacuo and 40 ml of water was added to the residue. Then the product was extracted with chloroform. The extract was dried and concentrated to dryness in vacuo. The residue was recrystallized from ethanol to provide 550 mg of 9-fluoro-3-methyl-10-(4methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H-pyrido[1,2,3de][1,4]benzoxazine-6-carboxylic acid as colorless needles with m.p. 250°257°C (with decomposition).
Therapeutic Function
Antibacterial
OFLOXACIN Preparation Products And Raw materials
Raw materials
Preparation Products
OFLOXACIN Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30240 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15839 | 69 |
| XiaoGan ShenYuan ChemPharm co,ltd | 15527768850 | 1791901229@qq.com | China | 8843 | 52 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12335 | 58 |
| Hubei XinyuanShun Chemical Co., Ltd. | 13971561712, 13995564702, 027-50664929 | hbeixys2001@163.com | China | 3120 | 55 |
| Shanghai Orgchem Co.,Ltd. | +86-21-5877 1921 | info@chemofchina.com | China | 9653 | 55 |
| PI & PI BIOTECH INC. | 020-81716320 17788709170 | Sales@pipitech.com | China | 2649 | 58 |
| PCL - Business, Laboratory Pharmaceutical | 19150123681 | Hu.xinyue@purechemland.com | China | 3566 | 58 |
83380-47-6(OFLOXACIN)Related Search:
1of2