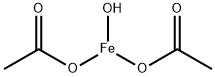
FERRIC ACETATE
- CAS No.
- 10450-55-2
- Chemical Name:
- FERRIC ACETATE
- Synonyms
- Basic ferric acetate;IRON (III) ACETATE;NSC 112208;FERRIC ACETATE;Iron hydroxyacetate;FERRIC ACETATE BASIC;Hydroxydiacetyl iron;Bis(acetato)hydroxyiron;Hydroxybis(acetato)iron;Iron hydroxide diacetate
- CBNumber:
- CB2164120
- Molecular Formula:
- C4H7FeO5
- Molecular Weight:
- 190.94
- MDL Number:
- MFCD00054407
- MOL File:
- 10450-55-2.mol
- MSDS File:
- SDS
| solubility | insoluble in H2O; soluble in ethanol, acid solutions |
|---|---|
| form | brown-red amorphous powder |
| color | brown-red amorphous powder |
| Water Solubility | insoluble H2O; soluble EtOH, acid [CRC10] |
| CAS DataBase Reference | 10450-55-2 |
| FDA UNII | A5CF0H2UXG |
FERRIC ACETATE Chemical Properties,Uses,Production
Description
Ferric acetate is the coordination compound more commonly known as "basic iron acetate". With the formula [Fe3O(OAc)6(H2O)3]OAc (OAc is CH3CO2-), it is a salt, composed of the cation [Fe3(μ3-O)(OAc)6(H2O)3]+ and an acetate anion . The formation of the red-brown complex has long been used as a test for ferric ions.
Chemical Properties
Red powder. Soluble in alcohol and acids; insoluble in water. Combustible.
Physical properties
Basic iron acetate forms on treating aqueous solutions of iron (III) sources with acetate salts.
Early work showed that it is trinuclear.The Fe centres are equivalent, each being octahedral, being bound to six oxygen ligands, including a triply bridging oxide at the center of the equilateral triangle.The compound was an early example of a molecular compound of iron that features an oxide ligand. Ignoring its 24 hydrogen centres, the cation has D3h symmetry.
Uses
In the textile industry as a mordant in dyeing and printing, and for the weighting of silk and felt; as wood preservative; in leather dyes.
Uses
Materials prepared by heating iron, acetic acid, and air, loosely described as basic iron acetates, are used as dyes and mordants.
Reactions
The terminal aqua ligands on the trimetallic frame work can be substituted with other ligands, such as pyridine and dimethyl formamide . Many different salts are known by exchanging the anion, e.g. [Fe3(μ3-O)(OAc)6(H2O)3]Cl. Reduction of the cation affords the neutral mixed-valence derivative that contains one ferrous and two ferric centers. Mixed metal species are known such as [Fe2CoO(OAc)6(H2O)3].
FERRIC ACETATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23541 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9639 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 2904 | 58 |
| Alfa Chemistry | Info@alfa-chemistry.com | United States | 24072 | 58 | |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49978 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |