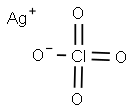SILVER PERCHLORATE
- CAS No.
- 7783-93-9
- Chemical Name:
- SILVER PERCHLORATE
- Synonyms
- SILVER PERCHLORATE;SILVER (I) PERCHLORATE;SILVER PERCHLORATE 99.9%;Perchloricacid,silver(1+)salt;Silver(I)perchlorate,anhydrous;perchloricacid,silver(1++)salt;Hyperchloric acid silver(I) salt;SILVER PERCHLORATE, ANHYDROUS, 97%;Silverperchlorate,anhydrous,min.97%;SILVER PERCHLORATE, ANHYDROUS REAGENT
- CBNumber:
- CB2180173
- Molecular Formula:
- AgClO4
- Molecular Weight:
- 207.32
- MDL Number:
- MFCD00003400
- MOL File:
- 7783-93-9.mol
- MSDS File:
- SDS
| Melting point | 486°C |
|---|---|
| Density | 2.8 g/mL at 25 °C(lit.) |
| solubility | benzene: slightly soluble(lit.) |
| form | Crystals |
| Specific Gravity | 2.806 |
| color | White |
| Water Solubility | Soluble in water and many organic solvents (benzene, toluene, pyridine)Soluble in water, benzene and toluene. |
| Sensitive | Hygroscopic |
| Merck | 14,8523 |
| Exposure limits |
ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.01 mg/m3 |
| Stability | hygroscopic |
| InChIKey | YDHABVNRCBNRNZ-UHFFFAOYSA-M |
| CAS DataBase Reference | 7783-93-9(CAS DataBase Reference) |
| FDA UNII | SV4X1U113O |
| EPA Substance Registry System | Perchloric acid, silver(1+) salt (7783-93-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H314 | |||||||||
| Precautionary statements | P210-P220-P260-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | O,C | |||||||||
| Risk Statements | 8-34 | |||||||||
| Safety Statements | 17-26-27-36/37/39 | |||||||||
| RIDADR | UN 1481 5.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 3-8-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
SILVER PERCHLORATE price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 674583 | Silver perchlorate anhydrous, 97% | 7783-93-9 | 5g | $70.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 674583 | Silver perchlorate anhydrous, 97% | 7783-93-9 | 25g | $249 | 2024-03-01 | Buy |
| Alfa Aesar | 042209 | Silver perchlorate, anhydrous | 7783-93-9 | 5g | $61.2 | 2024-03-01 | Buy |
| Alfa Aesar | 042209 | Silver perchlorate, anhydrous | 7783-93-9 | 25g | $223 | 2024-03-01 | Buy |
| Strem Chemicals | 93-4722 | Silver perchlorate, anhydrous, min. 97% | 7783-93-9 | 5g | $63 | 2024-03-01 | Buy |
SILVER PERCHLORATE Chemical Properties,Uses,Production
Description
Silver perchlorate (chemical formula: AgClO4) is a silver salt of perchloric acid as both a useful source of the Ag+ ion and a catalyst in the organic chemistry. It should be noted that due to the high instability and explosiveness of the anion perchlorate contained in it, it is limited for usage in industry. It is a kind of strong oxidizer and can be used to promoting the oxidation reaction in the organic synthesis. It can also be used as a catalyst for which it can be used to replace a halide with perchlorates to form intermediate in synthesis. It can be manufactured through the heating reaction of the mixture of perchloric acid with silver nitrate, the reaction between barium perchlorate and silver sulfate, or the reaction of perchloric acid with silver oxide.
References
https://en.wikipedia.org/wiki/Silver_perchlorate
http://www.softschools.com/formulas/chemistry/silver_perchlorate_formula/393/
Chemical Properties
The silver perchlorate salt, is deliquescent and forms a light-sensitive monohydrate that can be dehydrated at 43 °C and is soluble in a variety of organic solvents. Explosions of silver perchlorate have been reported.
Chemical Properties
Colorless crystals. Soluble in water; reacts with explosive violence with many organic sol- vents; explodes on grinding.
Uses
In the explosives industry.
Uses
Silver perchlorate is used as a catalyst in organic chemistry. It is an effective reagent for replacing halides ligands with perchlorate. It is also used in the manufacturing of explosives.
Purification Methods
Reflux it with *benzene (6mL/g) in a flask fitted with a Dean and Stark trap until all the water is removed azeotropically (ca 4hours). The solution is cooled and diluted with dry pentane (4mL/g of AgClO4). The precipitated AgClO4 is filtered off and dried in a desiccator over P2O5 at 1mm for 24hours [Radell et al. J Am Chem Soc 83 3958 1961]. It has also been recrystallised from perchloric acid. [Caution due to its EXPLOSIVE nature in the presence of organic matter.] Store it in the dark.
SILVER PERCHLORATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30240 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9414 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52925 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |