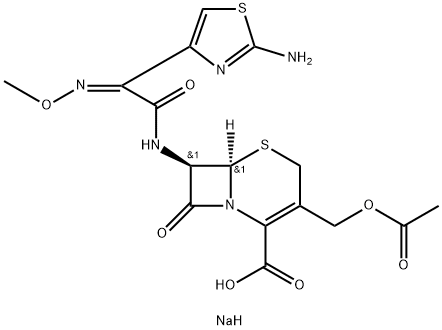
Cefotaxime sodium
- CAS No.
- 64485-93-4
- Chemical Name:
- Cefotaxime sodium
- Synonyms
- CEFOTAXIME SODIUM STERILE;ctx;CEFOTAXIME SODIUM SALT;CLAFORAN;Sodium cefotaxime;CEFOTAXIME NA-SALT;CEFOTAXIM SODIUM SALT;Cefotaxime Sodium (350 mg);Cefotaxime for peak identification;Cefotaxime, Sodium Salt, High Purity, Cell Culture-Tested
- CBNumber:
- CB2197478
- Molecular Formula:
- C16H18N5NaO7S2
- Molecular Weight:
- 479.46
- MDL Number:
- MFCD00079073
- MOL File:
- 64485-93-4.mol
| Melting point | 162-163 C |
|---|---|
| alpha | D20 +55±2° (c = 0.8 in water) |
| refractive index | 61 ° (C=1, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: aqueous solutions of pH 4.3-6.2 are stable up to 3 weeks at 2-8 °C.soluble |
| form | powder |
| color | white to yellow |
| PH | pH(100g/l, 25℃) : 4.5~6.5 |
| Water Solubility | Soluble in water. |
| Merck | 14,1933 |
| BRN | 5711411 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| NCI Dictionary of Cancer Terms | CTX |
| FDA UNII | 258J72S7TZ |
| NCI Drug Dictionary | Claforan |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P280-P284-P304+P340-P333+P313-P342+P311 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-60-45-37-24 |
| WGK Germany | 2 |
| RTECS | XI0250000 |
| HS Code | 29419000 |
Cefotaxime sodium price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2099 | Cefotaxime Sodium Pharmaceutical Secondary Standard; Certified Reference Material | 64485-93-4 | 1g | $160 | 2024-03-01 | Buy |
| Sigma-Aldrich | C7039 | Cefotaxime sodium salt plant cell culture tested, BioReagent, powder | 64485-93-4 | 1g | $291 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1097909 | Cefotaxime sodium United States Pharmacopeia (USP) Reference Standard | 64485-93-4 | 500mg | $436 | 2024-03-01 | Buy |
| Alfa Aesar | J62690 | Cefotaxime sodium salt | 64485-93-4 | 1g | $108 | 2024-03-01 | Buy |
| Alfa Aesar | J62690 | Cefotaxime sodium salt | 64485-93-4 | 5g | $220 | 2024-03-01 | Buy |
Cefotaxime sodium Chemical Properties,Uses,Production
Brand Name(s) in US
Claforan
Description
Cefotaxime was synthesized by Hoechst and Roussel-Uclaf in 1977. It was the first derivative of cephalosporin to introduce the methoxyimino and aminothiazole groups at the 7 position of the cephem nucleus. Although it shows unexpectedly low oral absorption, its excellent activity against a wide range of gram-positive and gram-negative organisms, including Serratia, Enterobacter, Citrobacter, and anaerobes, guided research and development of the newer synthetic cephems, the so-called thirdgeneration cephalosporins.
Chemical Properties
White to pale yellow crystalline powder
Originator
Claforan,Hoechst-Roussel,W. Germany,1980
Uses
Cefotaxime sodium salt acts as a beta-lactamase resistant antibiotic. It is used as an effective antibacterial against gram-negative bacteria, with the notable exception of pseudomonas and penicillin-resistant strains of streptococcus pneumoniae. It is used to treat infections of the bones, joints, skin, respiratory tract and blood stream.
Uses
Cephalosporin antibacterial
Uses
Broad spectrum third generation cephalosporin antibiotic. The name Cefotaxime applies to the isomer having a syn-methoxy imino group
Definition
ChEBI: A cephalosporin organic sodium salt having acetoxymethyl and [2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side-groups.
Manufacturing Process
A solution of 8 g of sodium bicarbonate in about 20 ml of ethanol was progressively added to 45.55 g of pure 3-acetoxymethyl-7-[2-(2-amino-4- thiazolyl)-2-methoxyiminoacetamido]-ceph-3-eme-4-carboxylic acid in 100 ml of distilled water and another 80 ml of ethanol and 4.5 g of activated carbon were added thereto. The mixture was stirred for 5 minutes and was filtered. The filter was rinsed with ethanol and the filtrate was evaporated to dryness under reduced pressure. The residue was taken up in 100 ml of ethanol and evaporated to dryness again. The residue was dissolved in 100 ml of methanol and the solution was poured into 2 l of acetone. The mixture was vigorously stirred and was vacuum filtered. The recovered product was rinsed with acetone and then ether and dried under reduced pressure to obtain 43.7 g of a white product which rehydrated in air to obtain a final weight of 45.2 g of sodium 3-acetoxymethyl-7-[2-(2-amino-4-thiazolyl)-2- methoxyiminoacetamido]-ceph-3-eme-4-carboxylate.
brand name
Claforan (Sanofi Aventis).
Therapeutic Function
Antibiotic
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biological Activity
mic: <0.1 μg/ml for s. pneumoniaecefotaxime is a cephalosporin antibiotic.the cephalosporins, a class of β-lactam antibiotics originally derived from the fungus acremonium, are indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic.
Biochem/physiol Actions
Cefotaxim inhibits bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs) which inhibits the final transpeptidation step of peptidoglycan synthesis in bacterial cell walls. As a result, bacteria lyse due to cell wall autolytic enzymes.
Clinical Use
Cefotaxime (Claforan) was the first third-generationcephalosporin to be introduced. It possesses excellentbroad-spectrum activity against Gram-positive and Gramnegativeaerobic and anaerobic bacteria. It is more activethan moxalactam against Gram-positive organisms. Manyβ-lactamase–producing bacterial strains are sensitive to cefotaxime,including N. gonorrhoeae, Klebsiella spp., H. influenzae,S. aureus, and E. cloacae. Some, but not all,Pseudomonas strains are sensitive. Enterococci and Listeriamonocytogenes are resistant.
The syn-isomer of cefotaxime is significantly more activethan the anti-isomer against β-lactamase–producing bacteria.This potency difference is, in part, because of greaterresistance of the syn-isomer to the action of β-lactamases.The higher affinity of the syn-isomer for PBPs, however,may also be a factor.
Cefotaxime is metabolized in part to the less active desacetylmetabolite. Approximately 20% of the metaboliteand 25% of the parent drug are excreted in the urine. Theparent drug reaches the cerebrospinal fluid in sufficient concentrationto be effective in the treatment of meningitis.Solutions of cefotaxime sodium should be used within 24hours. If stored, they should be refrigerated. Refrigerated solutionsmaintain potency up to 10 days.
Veterinary Drugs and Treatments
In the United States, there are no cefotaxime products approved for veterinary species but it has been used clinically in several species when an injectable 3rd generation cephalosporin may be indicated.
in vitro
previous studies found that the in-vitro activity of cefotaxime against staphylococcus au reus has ranged from 0.8 g to 8 μg/ml with 50% of isolates inhibited by 2 μg/ml and 90% by 4 μg/ml. moreover, it was found that methicillin resistant s. aureus were resistant to cefotaxime with mic values above 64 μg/ml. the cefotaxime mics against s. pneumoniae were found to be below 0.1 μg/ml, with 90% inhibited by 0.04 μg/ml. cefotaxime has also been shown to have excellent activity against haemophilus injluenzae, such as β-lactamase-containing strains [1].
in vivo
an in-vivo study with the mouse model of vibrio vulnificus infection was conducted to evaluate the efficacies of therapy with minocycline or cefotaxime alone and in combination. results indicated that combination therapy with cefotaxime and minocycline was distinctly more advantageous than therapy with the single antibiotic regimen for the treatment of severe vibrio vulnificus infections [2].
References
[1] neu hc. the in vitro activity, human pharmacology, and clinical effectiveness of new beta-lactam antibiotics. annu rev pharmacol toxicol. 1982;22:599-642.
[2] chuang yc, ko wc, wang st, liu jw, kuo cf, wu jj, huang ky. minocycline and cefotaxime in the treatment of experimental murine vibrio vulnificus infection. antimicrob agents chemother. 1998 jun;42(6):1319-22.
[3] schleupner cj, engle jc. clinical evaluation of cefotaxime for therapy of lower respiratory tract infections. antimicrob agents chemother. 1982 feb;21(2):327-33.
Cefotaxime sodium Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8811 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1015 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +86-18239973690 +86-18239973690 | sales@suikangpharm.com | China | 311 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 | admin@hblongbang.com | China | 941 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 | L18532138899@163.com | China | 939 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
View Lastest Price from Cefotaxime sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Cefotaxime sodium
64485-93-4
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2024-11-25 | Cefotaxime sodium
64485-93-4
|
US $0.00-0.00 / kg | 1kg | 99% | 20tons | Sinoway Industrial co., ltd. | |
 |
2024-11-25 | Cefotaxime sodium
64485-93-4
|
US $6.00 / kg | 1kg | More than 99% | 2000KG/Month | Hebei Longbang Technology Co., Ltd |
-

- Cefotaxime sodium
64485-93-4
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd
-

- Cefotaxime sodium
64485-93-4
- US $0.00-0.00 / kg
- 99%
- Sinoway Industrial co., ltd.
-

- Cefotaxime sodium
64485-93-4
- US $6.00 / kg
- More than 99%
- Hebei Longbang Technology Co., Ltd