ChemicalBook >> CAS DataBase List >>MANOOL
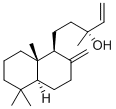
MANOOL
- CAS No.
- 596-85-0
- Chemical Name:
- MANOOL
- Synonyms
- MANOOL;Nsc165961;Manool (80%);(13R)-Labda-8(17),14-diene-13-ol;Labda-8(20),14-dien-13-ol, (13R)-;[13R,(+)]-Labda-8(17),14-dien-13-ol;[13R,(+)]-Labda-8(17),14-diene-13-ol;4ARTRANS-5-(-TETRAME-2-METHYLENEDECAHYDR;5-(5,5,8a-Trimethyl-2-methylenedecahydro-1-naphthalenyl)-3-methyl-1-penten-3-ol;(αR,1S,4aS,8aS)-α-Ethenyldecahydro-α,5,5,8a-tetramethyl-2-methylene-1-napthalenepropanol
- CBNumber:
- CB2334659
- Molecular Formula:
- C20H34O
- Molecular Weight:
- 290.48
- MDL Number:
- MFCD01310996
- MOL File:
- 596-85-0.mol
Last updated:2024-06-26 18:06:10
| Melting point | 49-52 °C(lit.) |
|---|---|
| Boiling point | 150-153 °C(Press: 0.3 Torr) |
| Density | 0.93±0.1 g/cm3(Predicted) |
| Flash point | >230 °F |
| storage temp. | 4°C, stored under nitroge |
| solubility | Benzene (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| pka | 14.47±0.29(Predicted) |
| form | Low-Melting Solid |
| color | White to Off-White |
| Specific Gravity | 0.97 |
| Odor | Very delicate, woody, dry-sweet, and extremely tenacious odor |
| LogP | 6.790 (est) |
| FDA UNII | AT5PJ0PV00 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H400-H319-H335 |
| Precautionary statements | P273-P391-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
MANOOL price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | E678100 | Manool(80%) | 596-85-0 | 100mg | $160 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FM65852 | Manool | 596-85-0 | 100mg | $200 | 2021-12-16 | Buy |
| AK Scientific | 9756AJ | Manool | 596-85-0 | 50mg | $218 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0003252 | MANOOL 95.00% | 596-85-0 | 100MG | $329.7 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FM65852 | Manool | 596-85-0 | 250mg | $350 | 2021-12-16 | Buy |
MANOOL Chemical Properties,Uses,Production
Uses
Maintains anti-proliferative activity against human malignant cell strains.
Preparation
By extraction of cornrninuted wood of Dacrydium biforme, a tree indigenous to New Zealand, with alcohol or Acetone. The extract is neutralized with alkali, and the crude Manool may be recrystallized.
Definition
ChEBI: A labdane diterpenoid in which the labdane skeleton has double bonds at positions 8(17) and 14 and carries an R-hydroxy group at position 13.
Cytotoxicity
IC50 (μg/mL): 49.3 (V79), 15.6 (B16F10),17.1 (MCF-7), 6.7 (HeLa), 28.5 (HepG2),9.6 (MO59 J), 6.7 (U343), 13.1 (U251)(de Oliveira et al. 2016)
MANOOL Preparation Products And Raw materials
MANOOL Suppliers
Global( 36)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49979 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| HBCChem, Inc. | +1-510-219-6317 | sales@hbcchem.com | United States | 10648 | 60 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 +86-13028896684; | sales@rrkchem.com | China | 57412 | 58 |
| BOC Sciences | -- | info@bocsci.com | USA | 0 | 65 |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 | sales@chemegen.com | China | 11217 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing@energy-chemical.com | China | 44918 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 15801484223; | psaitong@jm-bio.com | China | 29774 | 58 |
596-85-0(MANOOL)Related Search:
MANOOL
4AR-TRANS-5-(1,5,5,8AS-TETRAMETHYL-2-METHYLENEDECAHYDRO-1-NAPHTHALENYL)-3-R-METHYL-1-PENTEN-3-OL
1-Naphthalenepropanol, alpha-ethenyldecahydro-alpha,5,5,8a-tetramethyl-2-methylene-, [1S-[1alpha(S*),4abeta,8aalpha]]-
5-(5,5,8a-Trimethyl-2-methylenedecahydro-1-naphthalenyl)-3-methyl-1-penten-3-ol
Labda-8(20),14-dien-13-ol, (13R)-
4ARTRANS-5-(-TETRAME-2-METHYLENEDECAHYDR
1-Naphthalenepropanol, .alpha.-ethenyldecahydro-.alpha.,5,5,8a-tetramethyl-2-methylene-, (.alpha.R,1S,4aS,8aS)-
1-Naphthalenepropanol, .alpha.-ethenyldecahydro-.alpha.,5,5,8A-tetramethyl-2-methylene-, [1S-[1.alpha.(S*),4A.beta.,8A.alpha.]]-
Nsc165961
(αR,1S,4aS,8aS)-α-Ethenyldecahydro-α,5,5,8a-tetramethyl-2-methylene-1-napthalenepropanol
(13R)-Labda-8(17),14-diene-13-ol
(1S,4aα,αR)-Decahydro-α-vinyl-α,5,5,8aβ-tetramethyl-2-methylene-1-naphthalene-1-propanol
[13R,(+)]-Labda-8(17),14-dien-13-ol
[13R,(+)]-Labda-8(17),14-diene-13-ol
1-Naphthalenepropanol, α-ethenyldecahydro-α,5,5,8a-tetramethyl-2-methylene-, (αR,1S,4aS,8aS)-
Manool (80%)
(αR,1S,4aS,8aS)-α-Ethenyldecahydro-α,5,5,8a-tetramethyl-2-methylene-1-napthalenepropanol
596-85-0
Organic Building Blocks
Asymmetric Synthesis
Chiral Building Blocks
Alcohols
Miscellaneous Natural Products