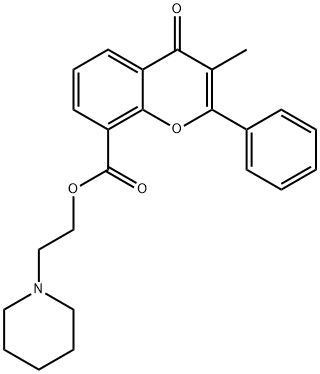
FLAVOXATE
- CAS No.
- 15301-69-6
- Chemical Name:
- FLAVOXATE
- Synonyms
- Uronid;Bladuril;FLAVOXATE;Flavoxate-d5;DW61 free base;FLAVOXATE USP/EP/BP;Rec-7-0040 free base;Piperidinoethyl 3-methyl-2-phenyl-4-oxo-4H-1-benzopyran-8-carboxylate;2-Piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate;2-(1-Piperidinyl)ethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate
- CBNumber:
- CB2341499
- Molecular Formula:
- C24H25NO4
- Molecular Weight:
- 391.46
- MDL Number:
- MFCD00210291
- MOL File:
- 15301-69-6.mol
| Melting point | 87-88 °C |
|---|---|
| Boiling point | 564.1±50.0 °C(Predicted) |
| Density | 1.203±0.06 g/cm3(Predicted) |
| pka | 7.3(at 25℃) |
| form | Off-white solid. |
| CAS DataBase Reference | 15301-69-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 3E74Y80MEY |
| ATC code | G04BD02 |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in rats (mg/kg): 1110 orally; 20.8 i.v. (Setnikar, 1975) |
|---|
FLAVOXATE price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Biosynth Carbosynth | BF164464 | Flavoxate | 15301-69-6 | 50mg | $43 | 2021-12-16 | Buy |
| Biosynth Carbosynth | BF164464 | Flavoxate | 15301-69-6 | 250mg | $105 | 2021-12-16 | Buy |
| AK Scientific | E125 | Flavoxate | 15301-69-6 | 50mg | $108 | 2021-12-16 | Buy |
| AK Scientific | E125 | Flavoxate | 15301-69-6 | 250mg | $191 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | RDL0006835 | FLAVOXATE-D5 95.00% | 15301-69-6 | 5MG | $502.15 | 2021-12-16 | Buy |
FLAVOXATE Chemical Properties,Uses,Production
Originator
Urispas,SKF,US,1971
Uses
Relaxant (smooth muscle).
Uses
Flavoxate is a pharmaceutical active-containing film delivery device for oral transmucosal administration.
Definition
ChEBI: A carboxylic ester resulting from the formal condensation of 3-methylflavone-8-carboxylic acid with 2-(1-piperidinyl)ethanol.
Manufacturing Process
A mixture of 13.3 grams of anhydrous aluminum chloride and 100 ml of
carbon disulfide is added to 19.4 grams of 2-propionyloxybenzoic acid
(prepared from the reaction of propionyl chloride and 2-hydroxybenzoic acid).
After an initial evolution of hydrogen chloride, the solvent is removed by
distillation and the mixture is heated at 150° to 160°C for 4 hours. The cooled
reaction mixture is treated with ice and hydrochloric acid and the product, 2-
hydroxy-3-carboxypropiophenone, is obtained from the oily residue by
distillation in vacuo.
A mixture of 1.9 grams of 2-hydroxy-3-carboxypropiophenone, 5.0 grams of
sodium benzoate and 20.0 grams of benzoic anhydride is heated at 180° to
190°C for 6 hours. A solution of 15.0 grams of potassium hydroxide in 50 ml
of ethanol and 20 ml of water is added and refluxed for 1 hour. The mixture is
evaporated and the residue after addition of water yields 3-methylflavone-8-
carboxylic acid.
To a suspension of 12.0 grams of 3-methylflavone-8-carboxylic acid in 200 ml
of anhydrous benzene is added 10.0 grams of thionyl chloride. The mixture is
refluxed for 2 hours during which the suspended solid goes into solution. The
solvent is completely removed by distillation, the residue extracted with
benzene and the extract evaporated to dryness. The product, 3-
methylflavone-8-carboxylic acid chloride, is recrystallized from ligroin to give
crystals melting at 155° to 156°C.
To 11.0 grams of 3-methylflavone-8-carboxylic acid chloride dissolved in 150
ml of anhydrous benzene is added at room temperature 4.8 grams of
piperidinoethanol and the mixture refluxed for 2 to 3 hours. The separated
solid is filtered, washed with benzene and dried. The product, piperidinoethyl
3-methylflavone-8-carboxylate hydrochloride is obtained as a colorless
crystalline solid, MP 232° to 234°C, (from US Patent 2,921,070).
brand name
Urispas (Ortho-McNeil).
Therapeutic Function
Spasmolytic
FLAVOXATE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569262 +86-15003564040 | 1087@dideu.com | China | 3167 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49980 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32343 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23912 | 58 |