Tin tetrachloride
- CAS No.
- 7646-78-8
- Chemical Name:
- Tin tetrachloride
- Synonyms
- SnCl4;TIN CHLORIDE;STANNIC CHLORIDE;Etain;Stagno;Fascat 4400;Tin(IV) chL;Ttin chloride;tinperchloride;Tetrachlorotin
- CBNumber:
- CB2381749
- Molecular Formula:
- Cl4Sn
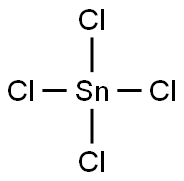
Lewis structure

- Molecular Weight:
- 260.52
- MDL Number:
- MFCD00011242
- MOL File:
- 7646-78-8.mol
- MSDS File:
- SDS
| Melting point | -33 °C (lit.) |
|---|---|
| Boiling point | 114 °C (lit.) |
| Density | 2.226 g/mL at 25 °C (lit.) |
| vapor density | 9 (vs air) |
| vapor pressure | 10 mm Hg ( 10 °C) |
| refractive index | 1.512 |
| Flash point | 34 °F |
| storage temp. | Store at RT. |
| solubility | Miscible with alcohol, benzene, toluene, chloroform, acetone, carbon terachloride, gasoline and carbon disulfide. |
| form | Solution |
| Specific Gravity | 2.226 |
| color | Colorless |
| PH | 0.2 (60g/l, H2O, 20℃)Hydrolysis |
| Water Solubility | reacts |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Sensitive | Air Sensitive |
| Merck | 14,8774 |
| Dielectric constant | 3.2(22℃) |
| Exposure limits |
ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Stability | Stability Stable, but may decompose upon exposure to moist air or water. Incompatible with strong bases, alcohols. |
| LogP | -1.67 at 20℃ |
| Substances Added to Food (formerly EAFUS) | STANNIC CHLORIDE |
| CAS DataBase Reference | 7646-78-8(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 67H76LFL3V |
| NIST Chemistry Reference | Tin tetrachloride(7646-78-8) |
| EPA Substance Registry System | Stannane, tetrachloro- (7646-78-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H335-H412 | |||||||||
| Precautionary statements | P261-P271-P273-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C,T,F,N | |||||||||
| Risk Statements | 34-52/53-40-23/24/25-11-67-37-65-50/53 | |||||||||
| Safety Statements | 26-45-61-7/8-36/37-24/25-23-36/37/39-16-62 | |||||||||
| RIDADR | UN 3264 8/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XP8750000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28273990 | |||||||||
| NFPA 704 |
|
Tin tetrachloride price More Price(45)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 208930 | Tin(IV) chloride 98% | 7646-78-8 | 250g | $81.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208930 | Tin(IV) chloride 98% | 7646-78-8 | 2kg | $386 | 2024-03-01 | Buy |
| TCI Chemical | T2053 | Tin(IV) Chloride (ca. 1.0mol/L in Dichloromethane) | 7646-78-8 | 100mL | $62 | 2024-03-01 | Buy |
| Alfa Aesar | 011571 | Tin(IV) chloride, anhydrous 98% (metals basis) | 7646-78-8 | 500g | $98.4 | 2024-03-01 | Buy |
| Alfa Aesar | 011571 | Tin(IV) chloride, anhydrous 98% (metals basis) | 7646-78-8 | *4x500g | $225 | 2023-06-20 | Buy |
Tin tetrachloride Chemical Properties,Uses,Production
Chemical Properties
Tin tetrachloride is a colorless fuming liquid with a pungent odor. Tin tetrachloride is soluble in cold water,alcohol,carbon disulfide, and oil of turpentine, that is decomposed by hot water to form hydrochloric acid with the evolution of heat. Tin tetrachloride is corrosive to metals and tissue.Used as a conductive coating and a sugar bleach,and in drugs, ceramics, soaps,and blue printing.
Uses
Tin(IV) chloride is a mordant for dying fabrics; a stabilizer for perfume in soap; used in weighting silk; in ceramic coatings; in manufacturing blue print papers; and to produce fuchsin. Also, tin(IV) chloride is used in preparing many organotin compounds.
Preparation
Tin(IV) chloride is prepared by reacting tin or tin(II) chloride with chlorine:
Sn + 2Cl2 → SnCl4
SnCl2 + Cl2 → SnCl4
Description
Tin (IV) chloride appears as white crystals with a strong pungent chlorine odour. On heating, tin (IV) chloride decomposition emits acrid fumes. At room temperature, it is colourless and releases fumes on contact with air, giving a stinging odour. Stannic chloride was used as a chemical weapon during World War I. It is also used in the glass container industry for making an external coating that toughens the glass. Stannic chloride is used in chemical reactions with fuming (90%) nitric acid for the selective nitration of activated aromatic rings in the presence of unactivated ones. Tin (IV) chloride reacts violently with water or moist air to produce corrosive hydrogen chloride. Tin (IV) chloride reacts with turpentine, alcohols, and amines, causing fire and explosion hazard. It attacks many metals, some forms of plastic, rubber, and coatings.
Chemical Properties
Stannic chloride is a white to yellow powder with a faint odor of HCl.
Chemical Properties
Tin tetrachloride is a colorless fuming liquid.
Chemical Properties
Colorless, fuming, caustic liquid, that water converts into a crystalline solid, SnCl4?5H2O.Keep well stoppered. Soluble in cold water, alcohol, carbon disulfide; decomposed by hot water.
Physical properties
Colorless fuming liquid; corrosive; density 2.234 g/mL; freezes at -33°C; boils at 114.15°C; critical temperature 318.75°C; critical pressure 37.98 atm; critical volume 351 cm3/mol; soluble in cold water, evolving heat; decomposed by hot water; soluble in alcohol, benzene, toluene, chloroform, acetone and kerosene
The pentahydrate is a yellowish-white crystalline solid or small, fused lumps; faint odor of HCl; density 2.04 g/cm3; decmposes at 56°C; very soluble in water; soluble in ethanol.
Uses
Tin(IV) chloride is a precursor to prepare organotin compounds such as tetralkyltin and dialkyldichlorotin(IV), which find applications as catalysts and polymer stabilizers. As a Lewis acid catalyst, it is used in Fridel-Crafts reactions for alkylation and cyclization. It is involved in the selective nitration of aromatic compounds in the presence of fuming nitric acid. Furthermore, it is used to prepare tin(IV) oxide coating by sol-gel process.
Uses
Electroconductive and electroluminescent coatings, mordant in dyeing textiles, perfume stabilization, manufacture of fuchsin, color lakes, ceramic coatings, bleaching agent for sugar, stabilizer for certain resins, manufacture of blueprint and other sensitized papers, other tin salts, bacteria and fungi control in soaps
Preparation
Tin(II) chloride is prepared by dissolving tin in hydrochloric acid followed by evaporation of the solution and crystallization.
Definition
Often sold in the form of the double salt with sodium chloride: Na2SnCl6?H2O.
General Description
Stannic chloride (SnCl4) is a strong Lewis acid widely used as a promoter or catalyst in organic synthesis. It is soluble in most organic solvents.
Air & Water Reactions
Fumes in moist air. Reacts with water to form Hydrochloric Acid in dense white fumes [Merck 11th ed. 1989].
Reactivity Profile
Acidic salts, such as STANNIC CHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions (ethylene oxide polymerization). Combination of the chloride with turpentine is strongly exothermic, and may lead to ignition, [Mellor, 1941, Vol. 7, 446].
Hazard
Evolves heat on contact with moisture. Corrosive liquid
Health Hazard
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. Combustible by chemical reaction. Upon contact with moisture, considerable heat is generated. Violent reaction with K, Na, turpentine, ethylene oxide, alkyl nitrates. Dangerous; hydrochloric acid is liberated on contact with moisture or heat. When heated to decomposition it emits toxic fumes of Cl-. See also HYDROCHLORIC ACID.
Potential Exposure
Tin tetrachloride is used in the production of blueprints and electroconductive readings, as a bleaching agent for sugar and resin stabilizer.
Shipping
UN2440 Stannic chloride, pentahydrate, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
SnCl4 fumes in moist air due to formation of a hydrate. Fractionate it in a ground glass still and store it in the absence of air. Possible impurities are SO2 and HCl [Baudler in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 729 1963]. It forms a solid pentahydrate [10026-06-9] which smells of HCl and is obtained when the anhydrous salt is dissolved in a small volume of H2O. Also reflux it with clean mercury or P2O5 for several hours, then distil it under (reduced) N2 pressure into a receiver containing P2O5. Finally redistil it. Alternatively, distil it from Sn metal under vacuum in an all-glass system and seal off in large ampoules. SnCl4 is available commercially as 1M solutions in CH2Cl2 or hexane. HARMFUL VAPOURS.
Incompatibilities
Slowly forms hydrochloric acid in cold water; fast reaction in hot water and steam. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, water, turpentine, potassium, sodium, ethylene oxide; nitrates, alcohols, amines, chlorine, strong acids; strong bases. Attacks metals, rubbers and some plastics in the resence of moisture.
Waste Disposal
SnCl4: Pour onto sodium bicarbonate; spray with ammonium hydroxide while adding crushed ice; when reaction subsides, flush down drain.


Tin tetrachloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| Changzhou Zechong Biopharma Co., Ltd. | +undefined18621330623 | sales@zechongchem.com | China | 28 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8811 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9210 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 563 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
View Lastest Price from Tin tetrachloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Tin tetrachloride
7646-78-8
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2023-08-14 | Tin tetrachloride
7646-78-8
|
US $35000.00 / kg | 25kg | 99.99% | 20ton | Hebei Mojin Biotechnology Co., Ltd | |
 |
2020-03-08 | Tin tetrachloride
7646-78-8
|
US $2.00 / KG | 1KG | 99%min | 200kg | Career Henan Chemical Co |
-

- Tin tetrachloride
7646-78-8
- US $10.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Tin tetrachloride
7646-78-8
- US $35000.00 / kg
- 99.99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Tin tetrachloride
7646-78-8
- US $2.00 / KG
- 99%min
- Career Henan Chemical Co
7646-78-8(Tin tetrachloride)Related Search:
1of4





