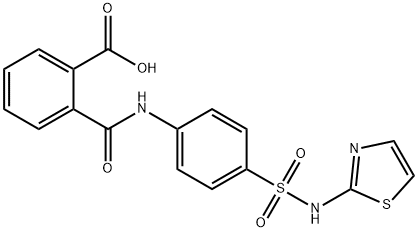
Phthalylsulfathiazole
- CAS No.
- 85-73-4
- Chemical Name:
- Phthalylsulfathiazole
- Synonyms
- PST;ST8SIA4;Talidine;Thalinil;MGC34450;MGC61459;Anti-PST;Ftalazol;Taloudron;Thalazole
- CBNumber:
- CB2405964
- Molecular Formula:
- C17H13N3O5S2
- Molecular Weight:
- 403.43
- MDL Number:
- MFCD00005318
- MOL File:
- 85-73-4.mol
- MSDS File:
- SDS
| Melting point | 198-204°C |
|---|---|
| Boiling point | 371.31℃[at 101 325 Pa] |
| Density | 1.4235 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.6390 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Practically insoluble in water, freely soluble in dimethylformamide, slightly soluble in acetone and in ethanol (96 per cent). |
| pka | 3.40±0.36(Predicted) |
| form | Solid |
| color | White to Light Yellow |
| Water Solubility | 400mg/L at 28℃ |
| Merck | 14,7377 |
| BRN | 359901 |
| LogP | -2 at 28℃ |
| CAS DataBase Reference | 85-73-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 6875L5852V |
| ATC code | A07AB02 |
| NIST Chemistry Reference | Phthalylsulfathiazole(85-73-4) |
| EPA Substance Registry System | Benzoic acid, 2-[[[4-[(2-thiazolylamino)sulfonyl]phenyl]amino]carbonyl]- (85-73-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H412 | |||||||||
| Precautionary statements | P273-P501 | |||||||||
| Safety Statements | 24/25 | |||||||||
| RIDADR | 3249 | |||||||||
| RTECS | TH8575000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29350090 | |||||||||
| Toxicity | LD50 i.p. in mice: 920 mg/kg (Mattis) | |||||||||
| NFPA 704 |
|
Phthalylsulfathiazole price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | WH0006817M1 | Monoclonal Anti-SULT1A1 antibody produced in mouse clone 1F8, ascites fluid | 85-73-4 | 200μL | $524 | 2024-03-01 | Buy |
| Sigma-Aldrich | P1500000 | Phthalylsulfathiazole European Pharmacopoeia (EP) Reference Standard | 85-73-4 | p1500000 | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | HPA053995 | Anti-ST8SIA4 antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody | 85-73-4 | 100μL | $580 | 2024-03-01 | Buy |
| Sigma-Aldrich | HPA051126 | Anti-ST8SIA4 antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution | 85-73-4 | 100μL | $580 | 2024-03-01 | Buy |
| Sigma-Aldrich | HPA049500 | Anti-SULT1A1 antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution | 85-73-4 | 100μL | $547 | 2024-03-01 | Buy |
Phthalylsulfathiazole Chemical Properties,Uses,Production
Chemical Properties
White or yellowish-white, crystalline powder.
Originator
Sulfathalidine, MSD, US,1946
Uses
Phthalylsulfathiazole is a sulfonamide antibacterial drug with similar and stronger effects than Sulfaguanidine and Succinylsulfathiazole. It is rarely absorbed orally and slowly decomposes in the intestine to release Sulfathiazole and exert intestinal antibacterial effects, and is used in the treatment of dysentery, colitis, and gastroenteritis.
Definition
ChEBI: Phthalylsulfathiazole is a sulfonamide incorporating 2-carboxybenzamido and 1,3-thiazol-2-yl moieties that is a broad-spectrum antibiotic indicated in the treatment of dysentery, colitis, gastroenteritis and intestinal surgery. It is a sulfonamide, a member of 1,3-thiazoles, a dicarboxylic acid monoamide and a sulfonamide antibiotic. It derives from a phthalic acid.
Preparation
It may be prepared by the interaction of sulfathiazole and phthalic anhydride in equimolar proportions.
It exerts its bacteriostatic effect in the gastro-intestinal tract. It has been found to be twice as active as sulfaguanidine in the treatment of bowel irregularities. It is often effective in watery diarrhoeas and ulcerative colitis. It is also used in the pre-operative treatment of patients undergoing surgery of the intestinal tract. It may also be recommended in the treatment of acute bascillary dysentry of the Sonne, Flexner and Shiga species.
Manufacturing Process
5 g of phthalic anhydride was added to a boiling suspension of 10 g of sulfathiazole in 100 cc of alcohol. The mixture was then refluxed for 5 minutes after the addition was complete at which time all of the solids were in solution. The solution was then cooled and diluted with an equal volume of water, The white solid precipitate which formed was filtered and recrystallized from dilute alcohol, yielding 2-N4-phthalylsulfanilamidothiazole,which decomposes above 260°C, according to US Patent 2,324,015.
brand name
Canidis-anti-diarr;Carbidiar;Carbotalin;Colicitina;Coliclase;Crematalil;Diacolin;Direver;Disenterol;Ef-micin;Enterocalme;Entero-hermes;Entero-red;Enterosteril;Entero-toxan;Esteraplidin mag;Eugeniteed;Fitazil;Ftalil-tiazol;Ilentazol;Inrestibla strepto;Iodentero-neomicina;Logical;Massotalil;Neo-sulfazon;Novosulfina;Phtazol;Septiftalil;Syptan;Talisulfazol;Tamil;.
Therapeutic Function
Antibacterial (intestinal)
World Health Organization (WHO)
Phthalylsulfathiazole, a sulfonamide anti-infective agent, was introduced in 1946 for the treatment of bacterial infections. The importance of sulfonamides has subsequently decreased as a result of increasing bacterial resistance and their replacement by antibiotics which are generally more active and less toxic. Although phthalylsulfathiazole, which is poorly absorbed from the gastrointestinal tract, is no longer recommended in some countries, it continues to be used in others for the treatment of local intestinal infections, including bacterial dysentery, and for pre-operative bowel preparation.
Flammability and Explosibility
Non flammable
Mode of action
Phthalylsulfathiazole is of low inherent toxicity. Besides, it also enjoys an additional plus point for being only slightly absorbed by the intestinal mucosa ; and, therefore, may be safely administered in comparatively large doses in the management and treatment of bacillary infections of the intestine. The drug is not absorbed orally and is mostly employed for ulcerative colitis.
Phthalylsulfathiazole Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei ZB Gamay Biological Technology Co.,Ltd | +8617330018573 | info@zbvet.net | China | 246 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | info@longyupharma.com | China | 2529 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
View Lastest Price from Phthalylsulfathiazole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Phthalylsulfathiazole
85-73-4
|
US $29.00 / mL | 95.58% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Phthalylsulfathiazole
85-73-4
|
US $29.00 / mL | 95.58% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Phthalysulfathiazole
85-73-4
|
US $0.00 / Box | 5Box | 99% | 200 | Hebei ZB Gamay Biological Technology Co.,Ltd |
-

- Phthalylsulfathiazole
85-73-4
- US $29.00 / mL
- 95.58%
- TargetMol Chemicals Inc.
-

- Phthalylsulfathiazole
85-73-4
- US $29.00 / mL
- 95.58%
- TargetMol Chemicals Inc.
-

- Phthalysulfathiazole
85-73-4
- US $0.00 / Box
- 99%
- Hebei ZB Gamay Biological Technology Co.,Ltd