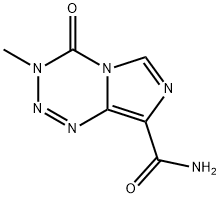
Temozolomide
- CAS No.
- 85622-93-1
- Chemical Name:
- Temozolomide
- Synonyms
- TMZ;Temozolamide;temodar;TeMozoloMid;TZM;methazolastone;M&B 39831;mb39831;Temodal
- CBNumber:
- CB2708199
- Molecular Formula:
- C6H6N6O2
- Molecular Weight:
- 194.15
- MDL Number:
- MFCD00866492
- MOL File:
- 85622-93-1.mol
- MSDS File:
- SDS
| Melting point | 212°C dec. |
|---|---|
| Boiling point | 526.6±42.0 °C(Predicted) |
| Density | 1.97±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble10mg/mL, clear |
| pka | 14.77±0.20(Predicted) |
| form | powder |
| color | white to light brown |
| Merck | 14,9139 |
| Stability | Stable for 2 years as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| InChIKey | BPEGJWRSRHCHSN-UHFFFAOYSA-N |
| CAS DataBase Reference | 85622-93-1(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Temodar; temozolomide |
| FDA UNII | YF1K15M17Y |
| NCI Drug Dictionary | Temodar |
| ATC code | L01AX03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H319-H335-H340-H350-H360FD | |||||||||
| Precautionary statements | P201-P202-P301+P312-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 45-46-60-61-22-36/37/38 | |||||||||
| Safety Statements | 24/25-45-36/37-26-53-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NJ5927050 | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
Temozolomide price More Price(61)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 5.00609 | Temozolomide - CAS 85622-93-1 - Calbiochem | 85622-93-1 | 50MG | $167 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1643543 | Temozolomide United States Pharmacopeia (USP) Reference Standard | 85622-93-1 | 200mg | $1030 | 2024-03-01 | Buy |
| Sigma-Aldrich | 34219 | Temozolomide VETRANAL , analytical standard | 85622-93-1 | 25mg | $428 | 2022-05-15 | Buy |
| TCI Chemical | T2744 | Temozolomide >98.0%(HPLC) | 85622-93-1 | 500mg | $52 | 2024-03-01 | Buy |
| TCI Chemical | T2744 | Temozolomide >98.0%(HPLC) | 85622-93-1 | 5g | $297 | 2024-03-01 | Buy |
Temozolomide Chemical Properties,Uses,Production
Anticancer drugs
Temozolomide is the first effective orally-taken imidazole and tetrazine-class anticancer drug which belongs to the second generation of an alkylating agent with antitumor activity without liver metabolic activation after oral administration. It is characterized by easily penetration through the blood-brain barrier, good tolerance and being not superimposed with other drugs toxicity, and having synergistic effect with radiotherapy which is suitable for treating malignant glioma recurrence after conventional treatment such as glioblastoma multiforme tumors or degenerative astrocytoma. It is first-line drug for treatment of metastatic melanoma.
Temozolomide had been first synthesized by Cancer Research UK Group, and then be transferred to the Schering-Plough Company (United States) for development. The drug is different from the existing Antineoplastic drug. It has a novel chemical structure and belongs to a four-imidazole derivative. Temozolomide does not play a direct role. At physiological pH, it is first quickly converted into active compound MTIC [5-(3-methyl-triazene-1-) imidazole-4-carboxamide] via non-enzymatically way. People think that the cytotoxicity of MTIC is mainly due to its DNA alkylation (methylation) effect. Alkylation mainly occurs in the O6 and N7 position of guanine. Basic and clinical studies of temozolomide have confirmed that it is effective in treating some of the most common glioma cells. In 1999, it has been approved for enter into market in EU and the US wherein the permitted indication in United States is mainly for second-line treatment of glioblastoma multiforme and degenerative star gliomas and approved indications of EU is for treating developing or relapsing glioblastoma multiforme which has already been subject to conventional therapy. The efficacy of temozolomide on treating glioblastoma multiforme has received more recognition in Europe.
Pharmacokinetics
This medicine can be completely oral absorbed and has a nearly 100% availability. It can exhibit a broad spectrum of activity in murine tumor model systems and can penetrate through the human blood-brain barrier. The toxic effects of temozolomide cells originate from its role in strong methylation of DNA bases. Under alkaline conditions, temozolomide can quickly be broken for formation of active methyl diazonium ion. Since the brain has a higher basicity than the surrounding tissue, so that the activation of this drug can relatively concentrate and occur at the tumor site. It has a strong anti-tumor effect with certain selectivity with its side-effect profile being alleviated, small bone marrow toxicity and improved patient tolerance.
Toxicological effects
Genetic Toxicity: Temozolomide has in vitro mutagenesis effect on bacteria (Ames test), and can cause the collapse of mammalian cell chromosomes (human peripheral blood lymphocytes clear test).
Reproductive toxicity: There currently have been no reproductive toxicity performed for temozolomide, but the toxicity study of repeated medication test of rat and dog have shown that when the administered doses of 50mg/m2 and 125mg/m2, for rat and dog, respectively (calculated by body surface area, equivalent to approximately 1/4 and 5/8 of maximum recommended dosage, respectively), this product is toxic to animal testicles exhibited as syncytial cells (i.e. immature sperm) occurs and testicular atrophy.
Carcinogenicity: There have been no conventional carcinogenicity studies about temozolomide yet. Giving 125 mg/m2 of temozolomide to rats in a cycle of 5 consecutive days per 28 days to (according to calculation of body surface area, it is comparable to the maximum recommended daily amount for human) for three cycles can produce breast cancer for both male and female rats. According to calculation of 25, 50, 125mg/m2 (according to the calculation of body surface area, it is roughly equivalent to 1/8 to 1/2 of the maximum recommended dosage) after administration of 6 cycles, the animals in all the dose group got breast cancer: at high-dose group, fibrosarcoma occurs in the heart, eyes, seminal vesicles, salivary glands, abdomen, uterus and prostate tissue. It is also appeared in seminal vesicle cancer, heart, nerve sheath tumors, optic nerve cancer, and Bernhard's adenocarcinoma. Moreover, adenoid tumor also occurs in other visible tissues such as animal skin, lung, pituitary, and thyroid.
The treatment of recurrent glioblastoma multiforme tumor
The treatment of Temozolomide for recurrent glioblastoma multiforme trials have confirmed upon clinical assays to be safe and effective. A randomized controlled trial in phase II have showed that 225 cases of glioblastoma multiforme patients suffering the first reoccurrence of pleomorphic glioblastoma who have been treated by temozolomide or procarbazine (every 56 days once every 28 d, oral administration of 125~150 mg/m2 per day) for 6 months, respectively, the temozolomide group of patients have got no disease deterioration with survival and overall survival rate being 21% and 60%, respectively. This was significantly higher than that in procarbazine group (8% and 44%). The average survival time and overall survival time for patients in the temozolomide group with no disease progression was 2.9 and 7.3 months, respectively, which were significantly higher than that in procarbazine group that was 1.9 and 5.8 months, respectively. The Experimental have also evaluated the health-related quality of life in both the first 3 months and 6 months after the starting of the treatment, the results also have confirmed that temozolomide group had more patients with quality of life being improved or remaining stable.
The main side effect for temozolomide is transient and non-retention bone marrow suppression which is both predictable and easy to handle with a trials incidence of 24%. Temozolomide is also prone to cause moderate nausea and vomiting in patients, however these adverse reactions can be prevented with conventional antiemetic therapy. The efficacy of temozolomide for treating recurrent glioblastoma multiforme efficacy and its property of adverse reaction were significantly better than the existing standard drug procarbazine.
Clinical evaluation
Temozolomide is effective in treating newly diagnosed glioblastoma multiforme cell tumor. A small II trial results has shown that 33 cases of such patients who had been subject to temozolomide (200mg/m2) treatment where 17 patients had achieved complete or partial remission, and other four patients were in stable condition. The average time from the disease remission to disease progression for 17 patients was seven months, and was two months for the treatment of non-responders with their average survival time being 12 months and 6 months. But the effect of temozolomide in treating newly diagnosed degenerative astrocytomas remains unclear.
Temozolomide is of high efficacy on glioma cells, especially has a significant effect on the most common pleomorphic glioblastoma. No matter in tumor response rates, or in patient survival and tolerability it all has a good effect. More importantly, the quality of patients’ life is significantly better than the existing standard drug procarbazine, moving a big step toward the treatment of tumors using this kind of drug. The first-line position of Temozolomide in treatment of progressive metastatic melanoma has been affirmed in clinical assays. Applications for applying it widely in Europe and the United States are being conducted. A recent randomized controlled trial results in III phase have shown about the 305 cases of these patients who subjected to temozolomide (200 mg/m2) and dacarbazine (every 21 days once a 5d, 250 mg/m2 per day) treatment, temozolomide group of patients had a overall survival and treatment response rates of 7.9 months and 13.5%, respectively, which was better than that of 5.7 months and 12.1% as observed in dacarbazine group. Patients in Temozolomide group had a better tolerance than dacarbazine group with its life-qualify evaluation (reflected by reduced physiological function) also significantly better than dacarbazine group (the rate of decline was 18% and 42% after three months of treatment, respectively). Melanoma skin tumors are rare skin cancer, even though it accounts for only about 4% of all skin cancers, but the mortality rate accounted for about 79% of all skin cancer. The current standard drug for treating it is still dacarbazine. Temozolomide has exhibited high clinical efficacy towards glial cells and melanoma, and thus is worthy of attention.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Patent No
DE 3237255; US 5260921
Description
Temozolomide was launched for the first time in the UK for the
treatment of patients with glioblastoma multiforme showing recurrence or
progression after standard therapy. It can be considered as a cyclic variant of
highly reactive triazenes producing a cascade of ionic or radical antitumoral
species. Temozolamide is a 3-methyl analog of mitozolomide and was shown to
be converted to cytotoxic triazene MCTIC. It can be prepared in 2 steps from 5-
aminoimidazole-4-carboxamide by diazotization then cyclization with
methylisocyanate. Mechanistically, the depletion of O6-alkylguanine-DNA
alkyltransferase (OGAT) in cells or tumors was shown to be correlated with the
cytotoxicity of temozolomide which is a potent inhibitor of this enzyme involved
in DNA repair activity.
Further approval applications for the treatment of other malignant gliomas such
as relapsed anaplastic astrocytoma, advanced metastatic melanoma were
submitted.
Chemical Properties
Off-white to light-pink crystalline solid, crystallized from dichloromethane, melting point 212℃ (decomposition). Maximum UV absorption (in 95% ethanol): 327nm.
Originator
CRC Technology (UK)
Uses
Temozolomide is an antineoplastic drug and an Imidazotetrazine alkylating agent. It induces apoptosis and causes cell cycle arrest at the G2/M checkpoint. It rapidly degrades in the body, producing the active metabolite MTIC, which exerts an anti-tumor effect. Moreover, temozolomide has shown effectiveness against paclitaxel-resistant tumors and has been utilized in the study of drug resistance mechanisms in glioblastoma cell lines.
Preparation
The synthesis of temozolomide involves several steps. First, 5-Amino-4-imidazolecarboxamide is diazotized using nitrite. Then, the diazotized compound is reacted with methyl isocyanate in dichloromethane. This reaction leads to the cyclization of the compound, ultimately resulting in the formation of temozolomide.
Definition
ChEBI: An imidazotetrazine that is 3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine which is substituted at positions 3, 4, and 8 by methyl, oxo, and carboxamide groups, respectively. A prodrug for MTIC (5-(3-methyltriaz-1-en-1-yl)-1H-i idazole-4-carboxamide, formed by spontaneous hydrolysis of temozolomide in the body), it is used as an oral alkylating agent for the treatment of newly diagnosed malignant glioblastoma multiforme (concomitantly with radiotherapy) and malignant melanoma.
Manufacturing Process
Reaction of 1H-imidazole-4-carboxilic acid amide with nitrous acid leads to the diazonium salt (5-diazenyl-1-H-imidazole-4-carboxilic acid amide). Condensation of the diazonium salt with methylisocyanate leads to initial formation of unstable urea which cyclizes under the reaction condition to give 3,4-dihydro-3-methyl-4-oxoimidazo(5,1-d)-1,2,3,5-tetrazine-8-carboxamide (temozolomide).
brand name
Temodar (Schering);Temodal.
Therapeutic Function
Antineoplastic
General Description
Temozolomide can be called as an oral alkylating agent and antitumour drug, which may show potent activity in the treatment of recurrent gliomas or glioblastoma, a kind of tumour that affects the brain or spine.
Biological Activity
DNA methylating, chemotherapeutic agent. Displays antitumor activity against a board spectrum of tumors, including leukemias, lymphomas and solid tumors (IC 50 = 5.0 μ M for cytotoxicity against mouse TLX5 lymphoma cells).
Biochem/physiol Actions
Temozolomide is a DNA methylating agent and drug resistance-modifying agent; anti-tumor and anti-angiogenic. Temozolomide induces G2/M arrest and apoptosis through adduction of a methyl group to O6 position of guanine in genomic DNA and functional inactivation of DNA repair protein O(6)-alkylguanine DNA alkyltransferase (AGT) in base excision repair (BER) pathway.
Clinical Use
This imidazolotetrazine derivative is administered orally in capsule form for the treatment of glioblastoma multiforme or in patients with anaplastic astrocytoma who have not responded to procarbazine or the nitrosoureas.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Metabolism
Oral absorption is rapid and complete. The CYP450 enzymes are not extensively involved in temozolomide metabolism, and less than 6% of the drug is excreted unchanged in the urine. Women clear the drug less effectively than men and have a higher incidence of severe neutropenia and thrombocytopenia in the initial therapy cycle. Food decreases temozolomide absorption, and myelosuppression is the most significant adverse effect.
storage
Store at RT
References
1) Kurzen et al. (2003), Inhibition of angiogenesis by non-toxic doses of temozolomide; Anticancer Drugs, 14 515
2) Gunther et al. (2003), Temozolomide induces apoptosis and senescence in glioma cells cultured as multicellular spheroids; Br. J. Cancer, 88 463
3) Danson et al. (2001), Temozolomide: a novel oral alkylating agent; Curr. Opin. Expert Rev. Anticancer Ther., 10 13
4) Natsumeda et al. (2011), Induction of autophagy in temozolomide treated malignant gliomas; Neuropathology, 31 486
Temozolomide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Mesochem Technology Co.,Ltd | +8613651027935 | rachel@mesochem.com | China | 191 | 58 |
| Shanghai Huici Pharmaceutical Technology Co., LTD | +8618917134367 | lixing@pharmahuici.com | China | 133 | 58 |
| Shanghai Huici Pharmaceutical Technology Co., LTD | +8618917134367 | lixing@pharmahuici.com | China | 133 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 444 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
Related articles
- Side effects of Temozolomide (TMZ)
- Temozolomide is used to treat certain types of brain tumors. Temozolomide is in a class of medications called alkylating agent....
- Jan 26,2022
View Lastest Price from Temozolomide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Temozolomide
85622-93-1
|
US $0.00 / Kg/Bag | 50g | 99%min | 10kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Temozolomide
85622-93-1
|
US $42.00-82.00 / mg | 99.87% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Temozolomide
85622-93-1
|
US $42.00-82.00 / mg | 99.87% | 10g | TargetMol Chemicals Inc. |
-

- Temozolomide
85622-93-1
- US $0.00 / Kg/Bag
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Temozolomide
85622-93-1
- US $42.00-82.00 / mg
- 99.87%
- TargetMol Chemicals Inc.
-

- Temozolomide
85622-93-1
- US $42.00-82.00 / mg
- 99.87%
- TargetMol Chemicals Inc.