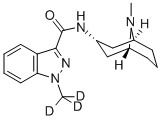
Granisetron
- CAS No.
- 109889-09-0
- Chemical Name:
- Granisetron
- Synonyms
- BRL 43964;BRL 43694;GRANISETRON;GRANESETRON;Granisteron;Gramisetron;Granisetron USP/EP/BP;granisetron(base,HCl);GRANISETRON(FREE BASE);Granisetron Impurity 22
- CBNumber:
- CB2728852
- Molecular Formula:
- C18H24N4O
- Molecular Weight:
- 312.41
- MDL Number:
- MFCD00865550
- MOL File:
- 109889-09-0.mol
| Boiling point | 532.0±40.0 °C(Predicted) |
|---|---|
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 25℃: DMSO |
| form | Powder |
| pka | 12.34±0.20(Predicted) |
| color | White to off-white |
| InChI | InChI=1/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12-,13+,14-/i2D3 |
| InChIKey | MFWNKCLOYSRHCJ-JQHPXDLNNA-N |
| SMILES | C1(C(=O)N[C@H]2C[C@@]3([H])CCC[C@@]([H])(C2)N3C)C2C=CC=CC=2N(C([2H])([2H])[2H])N=1 |&1:4,6,11,r| |
| NCI Dictionary of Cancer Terms | granisetron |
| FDA UNII | WZG3J2MCOL |
| NCI Drug Dictionary | granisetron |
| ATC code | A04AA02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H412-H302-H319 |
| Precautionary statements | P273-P501-P264-P270-P301+P312-P330-P501-P264-P280-P305+P351+P338-P337+P313P |
| HS Code | 2933399090 |
Granisetron price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1298092 | Granisetron United States Pharmacopeia (USP) Reference Standard | 109889-09-0 | 100MG | $460 | 2024-03-01 | Buy |
| TRC | G780013 | Granisetron | 109889-09-0 | 25mg | $75 | 2021-12-16 | Buy |
| ApexBio Technology | A3443 | Granisetron | 109889-09-0 | 50mg | $122 | 2021-12-16 | Buy |
| ApexBio Technology | A3443 | Granisetron | 109889-09-0 | 100mg | $208 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0005490 | GRANISETRON 95.00% | 109889-09-0 | 10MG | $280.35 | 2021-12-16 | Buy |
Granisetron Chemical Properties,Uses,Production
Chemical Properties
White to yellowish white crystalline powder, odorless, soluble in water, hard to dissolve in methanol, extremely hard to dissolve in ethanol, almost insoluble in ether. Melting Point: 290~292℃.
Anti-nausea drug
Launched by GSK's predecessor Britain SmithKline pharmaceutical company in the early 90s,Granisetron is an anti-nausea drug for the prevention and treatment of nausea and vomiting caused by chemotherapy and radiotherapy, with 6 ~ 11 time the anti-nausea effect of Ondansetron. Through blocking effect on the upper small intestine stomach centripetal nerve fibers and solitary nucleus or 5 - HT3 receptor in vomiting chemical feeling area, it suppresses nausea and vomiting caused by antitumor drugs and radiation.
Drug for chemotherapy-induced nausea and vomiting
Background
As one of the most important means of cancer treatment with positive effect, chemotherapy dose have many adverse reactions, among which vomiting is one of the most serious. The purpose of antiemetic therapy is to prevent or reduce the frequency and intensity of nausea and vomiting associated induced by chemotherapy. Early antiemetic drugs used in clinical practice have severe nerve center inhibition or extrapyramidal adverse reactions. Therefore, a variety of highly selective 5-ht3 receptor blockers have been developed since the 1980s and gradually develops into the mainstream drugs for the treatment of nausea and vomiting caused by chemotherapy. Based on the mechanism antiemetic drugs can be divided into dopamine blockers, vomiting central inhibitors, antihistamines, corticosteroids, 5-ht3 receptor blockers, and nk-1 receptor blockers.
5-HT3 receptor blocker
Since the launch of the first generation highly selective 5 - HT3 receptor blockers Ondansetron, a series of 5 - HT3 receptor blockers derivatives with determined curative effect and less adverse reaction have been used clinically, such as Granisetron, Navoban, Azasetron.
Mechanisms: the mechanism of chemotherapy induced nausea and vomiting is very complex which mainly includes the following aspects: the majority of cytotoxic drugs can stimulate the gastrointestinal mucosa causing mucosa damage and lead the chromaffin cells on mucous membrane, especially from the stomach to the ileal mucosa to release 5-HT which combines 5 - HT3 receptor to generate nerve impulses spreading to vomiting center forming vomiting; chemotherapeutic drugs and the metabolites stimulate and activate the vomiting center to form vomit; the various toxic substances in blood can act on the vomiting center unprotected by blood-brain barrier and the signal can be passed to vomiting center causing vomiting, the vomiting center reacts to a variety of stimuli which are activated through a series of receptors (dopamine receptors, histamine receptors, muscarine receptors, 5-HT3 receptors). Most antiemetic drugs also play a role in acting on one or more receptors. 5-HT is an important central transmitter in the human body. Its receptor is divided into 4 types,5-HT1, 5-HT2, 5-HT3, 5-HT4 and several subtypes. 5 - HT3 receptor blockers can be applied to the 5-HT3 receptors on vagus nerve to inhibit the excitement of vagal into fibers and inhibit the activation of AP and NTS 5 - HT3 by acting on the receptors, thus blocking afferent impulse to the vomiting center and inhibiting vomiting.
Nk-1 receptor blocker
Mechanism: P substance and its receptor are new targets for the treatment of nausea and vomiting drugs induced by chemotherapy. As a polypeptide containing 11 amino acids, Substance P along with neurokinin A (NKA) and neurokinin B (NKB) belongs to the tachykinin family which has three subtypes (NK 1 receptor, NK - 2 receptor, NK receptors - 3). P substance is mainly found in the central nervous system and gastrointestinal tract and has the strongest binding force with nk-1 receptor. There have been tests to prove that intravenous injection of P can cause vomiting, and the application of selective Nk-1 receptor blockers can block the vomiting caused by cytotoxic chemotherapy drugs.
Other Anti-nausea drugs
Aprepitant : usually in conjunction with other antiemetic drugs and can only be used to prevent nausea and vomiting caused by cancer drugs, noneffective for existing nausea and vomiting. Clinical studies show that NK- 1 receptor blockers aprepitant, along with 5-HT3 receptor blockers and dexamethasone, can increase the control ratio by 20% in acute vomiting, and by 30% ~ 40% in the delayed vomiting without drug tolerance in the later course of treatment.
The clinical trial phase Ⅲ of aprepitant show that on the basis of high dose of cisplatin, oral aprepitant (125 mg 1st day, 80 mg 2nd ~ 3rd day ) with
Ondansetron and Dexamethasone has better effect on acute and delayed vomiting than Ondansetron and Dexamethasone and good tolerance.
Casopitant: NK-1 receptor blocker developed by GlaxoSmithKline
According to the latest clinical data from casopitant(GW679769) for nausea and vomiting indications, casopitant has a better efficacy than ondansechin and matches arapitant if combined with ondansechin. The two dose phase Ⅱ clinical trials involving 1200 patients in the United States show that casopitant can be effectively used in moderate and severe vomint chemotherapy. In moderate vomit-induced chemotherapy trials, 85% of patients show completely curative effect with 150 mg casopitant used with dexamethasone and ondansechin , while the data is only 70% (P < 0.05) if dexamethasone and ondansechin are just used . In severe vomit-induced chemotherapy trials, 86% of patients have completely curative effect with 100mg casopitant and dexamethasone and ondansechin used, while the data is only 60%(P < 0.05) if dexamethasone and ondansechin are just used.
Pharmacokinetics
This product is widely distributed in the body, and the binding rate of serum protein is about 65%. The metabolic pathway is mainly conjugated after N dealkylation and aromatic epoxidation. It is excreted mainly through urine and feces.
Adverse effects
Human studies show that this product has good tolerance. Like other drugs, common adverse reactions are just headache and constipation, most of which are from mild to moderate. Occasionally allergic reaction happens of which is heavy sometimes (such as anaphylactic shock). Other allergic reactions include mild rash. In clinical trials, liver transaminase transient characteristic increases but still in normal range.
Taboo
Allergic to this product
Patients with gastrointestinal obstruction.
Precautions
- The injection preparation can be diluted with physiological saline and 5% glucose injection and prepared just when using. The diluted injection should not be stored for more than 24 hours with light avoidance and room temperature.
- The injection should not be mixed with other drugs before use.
- The does should not be more than 10mg daily to avoid further increase of blood pressure.
Medical interaction
- Dexamethasone can increase the efficacy
- In vitro studies have shown that ketoconazole may inhibit the metabolism of this product by acting on CYP 3A isozyme, but its clinical significance is unclear.
Uses
Anti-emetic.
Uses
Granisetron is a selective 5HT-3 antagonist.
Indications
granisetron (Kytril) is potent antagonists of 5-HT3 receptors,which is found peripherally on vagal nerve terminals and centrally in the CTZ. During chemotherapy that induces vomiting, mucosal enterochromaffin cells in the GI tract release serotonin, which stimulates 5-HT3 receptors.
Definition
ChEBI: A monocarboxylic acid amide resulting from the formal condensation of the carboxy group of 1-methyl-1H-indazole-3-carboxylic acid with the primary amino group of (3-endo)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine. A sele tive 5-HT3 receptor antagonist, it is used (generally as the monohydrochloride salt) to manage nausea and vomiting caused by cancer chemotherapy and radiotherapy, and to prevent and treat postoperative nausea and vomiting.
Clinical Use
Prevention or treatment of nausea and vomiting induced by cytotoxic chemotherapy, radiotherapy, or postoperative nausea and vomiting (PONV)
Side effects
This causes vagal afferent discharge, inducing vomiting. In binding to 5-HT3 receptors, granisetron blocks serotonin stimulation, hence vomiting, after emetogenic stimuli such as cisplatin. Headache is the most frequently reported adverse effect of these medications.
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: possible increased risk of ventricular
arrhythmias with panobinostat.
Metabolism
Granisetron is metabolised primarily in the liver by oxidation followed by conjugation. The major compounds are 7-OH-granisetron and its sulphate and glycuronide conjugates. Although antiemetic properties have been observed for 7-OH-granisetron and indazoline N-desmethyl granisetron, it is unlikely that these contribute significantly to the pharmacological activity of granisetron in man. Clearance is predominantly by hepatic metabolism. Urinary excretion of unchanged granisetron averages 12% of dose whilst that of metabolites amounts to about 47% of dose. The remainder is excreted in faeces as metabolites.
storage
+4°C (desiccate)
Granisetron Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | 3684455296@qq.com | China | 9300 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29860 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12132 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32159 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10244 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 7753 | 58 |
View Lastest Price from Granisetron manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-28 | Granisetron
109889-09-0
|
US $30.00 / kg | 1kg | 99% | 10ton | Shanghai Likang New Materials Co., Limited | |
 |
2021-07-13 | Granisetron
109889-09-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-09 | Granisetron
109889-09-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Granisetron
109889-09-0
- US $30.00 / kg
- 99%
- Shanghai Likang New Materials Co., Limited
-

- Granisetron
109889-09-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Granisetron
109889-09-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd