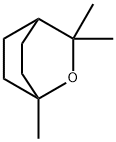
1,8-Cineole
- CAS No.
- 470-82-6
- Chemical Name:
- 1,8-Cineole
- Synonyms
- EUCALYPTOL;CINEOLE;CINEOL;1,8-CINEOL;MENTHANE;Eukalyptol;LABOTEST-BB LT00440774;1,3,3-trimethyl-2-oxabicyclo(2.2.2)octan;EucaL;Terpan
- CBNumber:
- CB2853653
- Molecular Formula:
- C10H18O
- Molecular Weight:
- 154.25
- MDL Number:
- MFCD00167977
- MOL File:
- 470-82-6.mol
- MSDS File:
- SDS
| Melting point | 1-2 °C(lit.) |
|---|---|
| Boiling point | 176-177 °C(lit.) |
| Density | 0.9225 |
| vapor pressure | 1.22hPa at 20℃ |
| FEMA | 2465 | EUCALYPTOL |
| refractive index |
n |
| Flash point | 122 °F |
| storage temp. | 2-8°C |
| solubility | 3.5g/l |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Odor | at 10.00 % in dipropylene glycol. eucalyptus herbal camphor medicinal |
| Odor Type | herbal |
| Viscosity | 3.4mm2/s |
| Water Solubility | Soluble in water(3500 mg/L (at 21°C). Miscible with ether, alcohol, chloroform, glacial acetic acid, oils. Soluble in ethanol, ethyl ether; slightly soluble in carbon tetrachloride. |
| FreezingPoint | min. 1.4 ℃ |
| JECFA Number | 1234 |
| Merck | 14,3895 |
| BRN | 105109 |
| Dielectric constant | 4.8399999999999999 |
| Stability | Stable. Flammable. Incompatible with acids, bases, strong oxidizing agents. |
| InChIKey | WEEGYLXZBRQIMU-WAAGHKOSSA-N |
| LogP | 3.4 |
| CAS DataBase Reference | 470-82-6(CAS DataBase Reference) |
| Substances Added to Food (formerly EAFUS) | EUCALYPTOL |
| FDA 21 CFR | 310.545 |
| EWG's Food Scores | 1 |
| FDA UNII | RV6J6604TK |
| ATC code | R05CA13 |
| NIST Chemistry Reference | Eucalyptol(470-82-6) |
| EPA Substance Registry System | Eucalyptol (470-82-6) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H317 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P280-P303+P361+P353 | |||||||||
| Hazard Codes | Xi,F | |||||||||
| Risk Statements | 10-37/38-41-36/37/38 | |||||||||
| Safety Statements | 26-39-16 | |||||||||
| RIDADR | UN 1993 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | OS9275000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2932 99 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| Hazardous Substances Data | 470-82-6(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 2480 mg/kg | |||||||||
| NFPA 704 |
|
1,8-Cineole price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W246506 | Eucalyptol natural, ≥99%, FCC, FG | 470-82-6 | 1kg | $115 | 2024-03-01 | Buy |
| Sigma-Aldrich | W246506 | Eucalyptol natural, ≥99%, FCC, FG | 470-82-6 | 10Kg | $1360 | 2024-03-01 | Buy |
| Sigma-Aldrich | W246506 | Eucalyptol natural, ≥99%, FCC, FG | 470-82-6 | 20kg | $1750 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHL89195 | Eucalyptol phyproof? Reference Substance | 470-82-6 | 100MG | $366 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM40684 | Eucalyptol solution certified reference material, 2000?μg/mL in methanol, ampule of 1?mL | 470-82-6 | 1mL | $102 | 2024-03-01 | Buy |
1,8-Cineole Chemical Properties,Uses,Production
Description
Eucalyptol is a bicyclic monoterpene that has been found in Eucalyptus and other plants, including C. sativa and has diverse biological activities, including anti-inflammatory, decongestant, antinociceptive, and insect repellent properties. Eucalyptol (10 μM) inhibits TNF-α, IL-1β, IL-4, and IL-5 production by primary human lymphocytes stimulated by ionomycin and phorbol 12-myristate 13-acetate (PMA; ). It also decreases LPS-induced mucus production by primary human nasal turbinate slices when used at a concentration of 10 μM. Eucalyptol (400 mg/kg) decreases carrageenan-induced hind paw edema in rats and reduces the time spent licking the hind paw in a formalin-induced nociception test in mice. It inhibits A. aegypti mosquitoes from feeding on anesthetized gerbils when applied topically at a concentration of 10% and from laying eggs in an ovipositional bioassay when used at a concentration of 1% in standing water. Formulations containing eucalyptol have been used in mouthwash and cough suppressants.
Chemical Properties
Eucalyptol has a characteristic camphoraceous odor and fresh, pungent, cooling taste.
Chemical Properties
1,8-Cineole occurs in many terpene-containing essential oils, sometimes as the main component. For example, eucalyptus oils contain up to 85% 1,8-cineole and laurel leaf oil contains up to 70%. It is a colorless liquid with a characteristic odor, slightly reminiscent of camphor. 1,8-Cineole is one of the few fragrance materials that is obtained exclusively by isolation from essential oils, especially eucalyptus oils. Technical-grade 1,8- cineole with a purity of 99.6–99.8% is produced in large quantities by fractional distillation of Eucalyptus globulus oil. A product essentially free from other products can be obtained by crystallization of cineole-rich eucalyptus oil fractions
Occurrence
Its name is derived from its presence in the essential oils of Eucalyptus globulus and Melaleuca leucadendron L. (essential oil of cajeput). It was originally identified in the essential oil of Artemisia maritime and subsequently in a large number (approx. 270) of other essential oils: rosemary, laurel leaves, clary sage, myrrh, cardamom, star anise, camphor, lavender, peppermint, Litsea guatemalensis, Luvunga scadens Roxb., Achillea micrantha and Salvia triloba. The essential oil of Eucalyptus polibrac tea has been reported to contain up to 91% eucalyptol. Also reported found in citrus oils and juices, guava, papaya, cinnamon bark, root and leaf, ginger, corn mint oil, spearmint, nutmeg, pepper, Thymus zygis, cardamom, cranberry, laurel, pepper, sweet marjoram, coriander, Spanish origanum, Ocimum basilicum, curcuma, sage, laurel, sweet and bitter fennel, myrtle leaf and berry, pimento and calamus.
Uses
eucalyptol is considered an antiseptic. This is a monoterpene compound that provides the fragrance associated with the essential oil of eucalyptus. eucalyptol is also used to fragrance cosmetic preparations.
Uses
Labelled 1,8-Cineol, the chief constituent of oil of eucalyptus. Used as pharmaceutic aid (flavor).
Uses
1,8-Cineol is the chief constituent of oil of eucalyptus. Used as pharmaceutic aid (flavor).
Preparation
By fractional distillation (170 to 180°C) from those essential oils containing high levels of eucalyptol, such as Eucalyptus globulus (approx. 60%), and subsequent separation of the product by congealing the distillate.
Definition
ChEBI: 1,8-cineole is a cineole. It has a role as a flavouring agent.
Aroma threshold values
Detection: 1 to 64 ppb. Aroma characteristics at 1.0%: sweet, cooling, fresh, chemical pine, slightly minty with a spicy cardamom nuance.
Taste threshold values
Taste characteristics at 5 ppm: cooling, fresh, oily, green, spicy, pine-like.
General Description
Colorless liquid with a camphor-like odor. Spicy cooling taste.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Cineole will react with acids and bases.
Fire Hazard
Flash point data for Cineole are not available but Cineole is probably combustible.
Flammability and Explosibility
Flammable
Biochem/physiol Actions
Taste at 30 ppm
Anticancer Research
A statistically significant reduction of cell proliferation was observed compared tothe control cells when tested on RKO cells and human colon cancer cell linesHCT116 injected into the SCID mice. The 1,8-cineole induced apoptosis by inactivatingsurviving and Akt, activating p38, inducing PARP, and cleaving caspase-3(Rodd et al. 2015).
Toxicology
Eucalyptol, a colourless organic compound, is a monoterpenoid and an ether. Among its various uses, eucalyptol is predominantly used as an insecticide and in fragrances (Klocke et al. 1987). The ques- tion of whether eucalyptol could potentiate toxicity has been assessed because of its widespread use in households. When Kunming mice received feed with a high dose of eucalyptol, liver and kidney tissue demonstrated vacuolar and granular degeneration (Xu et al. 2014). When the animals were fed with a subacute dose of eucalyptol, no discernible difference in body weight was observed (Xu et al. 2014). Eucalyptol’s safety profile has been assessed. Fatality after ingestion of eucalyptol oil has been reported; the approximate lethal dose of eucalyptol in the human is between 0.05 and 0.5 mL/kg of body weight (Hindle 1994). When male rats received eucalyptol, eosinophilic protein droplets accumulated in a dose- dependent fashion (Kristiansen and Madsen 1995). However, in a study of mutagenicity using CHO cells, eucalyptol did not induce mutagenicity as evidenced in the sister chromatid exchange assay (Galloway et al. 1987). Likewise, eucalyptol did not induce carcinogenicity in pathogen-free CFLP mice (Roe et al. 1979).
Metabolism
Eucalyptol undergoes oxidation in vivo with the formation of hydroxy cineole, which is excreted as hydroxycineoleglucuronic acid (Williams, 1959).
Solubility in organics
Soluble in Propylene glycol, miscible with alcohol and oils.
Purification Methods
Purify 1,8-cineol by dilution with an equal volume of pet ether, then saturate with dry HBr. The precipitate is filtered off, washed with small volumes of pet ether, then cineole is regenerated by stirring the crystals with H2O. It can also be purified via its o-cresol or resorcinol addition compounds. Store it over Na until required. Purify it also by fractional distillation. It is insoluble in H2O but soluble in organic solvents. [IR: Kome et al. Nippon Kagaku Zasshi [J Chem Soc Japan (Pure Chem Sect)] 80 66 1959, Chem Abstr 603 1961, Beilstein 17 II 32, 17/1 V 273.]
1,8-Cineole Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12809 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21628 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29859 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | scglp@glp-china.com | CHINA | 1824 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860; +8618080483897 | sales@biopurify.com | China | 3772 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2957 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 | sales@amoychem.com | China | 6383 | 58 |
Related articles
- Application and Toxicity of Cineole
- Cineole is a natural organic compound that is a colorless liquid. It is a cyclic ether and a monoterpenoid.
- Nov 18,2019
View Lastest Price from 1,8-Cineole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-24 | Cineole
470-82-6
|
US $5.00 / kg | 1kg | ≥99% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2025-04-23 | 1,8-Cineole
470-82-6
|
US $10.00 / KG | 1KG | 99% | 5tons | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-03-21 | 1,8-Cineole
470-82-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd |
-

- Cineole
470-82-6
- US $5.00 / kg
- ≥99%
- Jinan Finer Chemical Co., Ltd
-

- 1,8-Cineole
470-82-6
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- 1,8-Cineole
470-82-6
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd