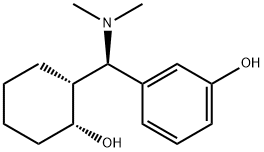
Ciramadol
- CAS No.
- 63269-31-8
- Chemical Name:
- Ciramadol
- Synonyms
- WY-15705;Ciramadol;Ciramadolum;Unii-9nq109ow0g;Ciramadolum [inn-latin];Phenol, 3-((R)-(dimethylamino)((1R,2R)-2-hydroxycyclohexyl)methyl)-;3-[(R)-(Dimethylamino)[(1R)-2α-hydroxycyclohexan-1α-yl]methyl]phenol
- CBNumber:
- CB31178305
- Molecular Formula:
- C15H23NO2
- Molecular Weight:
- 249.35
- MDL Number:
- MFCD00864377
- MOL File:
- 63269-31-8.mol
| Melting point | 191-193° |
|---|---|
| alpha | D25 -46.92° (c = 1.061 in methanol) |
| Boiling point | 385.7±17.0 °C(Predicted) |
| Density | 1.127±0.06 g/cm3(Predicted) |
| pka | 9.84±0.10(Predicted) |
| FDA UNII | 9NQ109OW0G |
Ciramadol Chemical Properties,Uses,Production
Originator
Ciramadol,American Home
Uses
Ciramadol is an opioid analgesic. It is a mixed agonist-antagonist for the μ-opioid receptor with relatively low abuse potential and a ceiling on respiratory depression.
Definition
ChEBI: Ciramadol is a member of cyclohexanols.
Manufacturing Process
m-Methoxymethoxybenzaldehyde (167 g, 1.0 mole) and cyclohexanone (318
ml, 3.0 moles) were refluxed for 4 hours under nitrogen with a solution of
potassium hydroxide (50 g, 0.89 moles) in water (1 liter). After cooling the
oily layer was extracted with ether (twice). The ether solution was washed
with water (thrice), brine, dried (sodium sulfate), and evaporated. The residue
was distilled and the product obtained as a yellow oil (132 g.), boiling point of
m-methoxymethoxybenzalcyclohexanone 173-176°C at 0.3 mm, λmax 287 nm
(ethanol, ε 13,000).
A solution of m-methoxybenzalcyclohexanone (50 g ) in ether (50 ml) was
cooled to -5°C in a pressure bottle and treated with 20 ml dimethylamine. The
bottle was stoppered and left at room temperature during 60 hours. The
above reaction was performed in duplicate (IR monitoring indicates the
mixture attains an equilibrium concentration in which the β-dimethylamino
ketone addition product is favored over the m-methoxybenzalcyclohexanone
by a ratio of ca. 2:1). the combined total reaction mixtures were added
dropwise under nitrogen to a stirred suspension of LiAlH4 (20 g)
in ether (1.2
liters) and the mixture was refluxed during 4 hours. The ice cooled reaction
mixture was treated with 3% aqueous sodium hydroxide solution (100 ml)
and filtered. The precipitated solids were washed with boiling ether and the
combined filtrates evaporated to approximately 1 liter. The ether layer was
extracted (twice) with an excess of dilute hydrochloric acid followed by a
water extraction. The combined aqueous extracts were back extracted with
ether, basified with 50% sodium hydroxide solution and extracted with ether
(twice). The ether layers were washed with brine and evaporated to an oil (50
g) to give a mixture containing predominantly (96%) of two components. 32 g
of the residue were chromatographed on a Woelm alumina column (900 g
Therapeutic Function
Analgesic
Ciramadol Preparation Products And Raw materials
Ciramadol Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.cn | China | 16377 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |