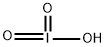Iodic acid
- CAS No.
- 7782-68-5
- Chemical Name:
- Iodic acid
- Synonyms
- iodic;10dicacid;Iodic aci;odic acid;IODIC ACID;IODIC ACID GR;Iodicacid,99%;IODIC ACID AR;Monoiodic acid;IODIC (V) ACID
- CBNumber:
- CB3132412
- Molecular Formula:
- HIO3
Lewis structure

- Molecular Weight:
- 175.91
- MDL Number:
- MFCD00011348
- MOL File:
- 7782-68-5.mol
- MSDS File:
- SDS
| Melting point | 110°C (dec.) |
|---|---|
| Density | 4.63 g/cm3 |
| solubility | Water (Slightly) |
| pka | 1.28±0.53(Predicted) |
| form | Solid |
| color | White to off-white |
| Specific Gravity | 4.629 |
| PH | pKa1= 0.70(25℃) |
| Water Solubility | 269 g/100 mL (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,5012 |
| Stability | Stable. Incompatible with strong reducing agents, alcohols, organic materials. Light-sensitive. |
| InChIKey | ICIWUVCWSCSTAQ-UHFFFAOYSA-N |
| CAS DataBase Reference | 7782-68-5(CAS DataBase Reference) |
| FDA UNII | 6U8J18JSBM |
| EPA Substance Registry System | Iodic acid (7782-68-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H314 | |||||||||
| Precautionary statements | P220-P280-P305+P351+P338-P310 | |||||||||
| Hazard Codes | O,C | |||||||||
| Risk Statements | 8-34 | |||||||||
| Safety Statements | 17-26-36/37/39-45 | |||||||||
| RIDADR | UN 3085 5.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28111980 | |||||||||
| NFPA 704 |
|
Iodic acid price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 58060 | Iodic acid puriss. p.a., ACS reagent, ≥99.5% (RT) | 7782-68-5 | 25g | $143 | 2024-03-01 | Buy |
| Sigma-Aldrich | 221929 | Iodic acid ACS reagent, ≥99.5% | 7782-68-5 | 50g | $173 | 2024-03-01 | Buy |
| Alfa Aesar | 087681 | Iodic acid, ACS, 99.5% | 7782-68-5 | 50g | $90.5 | 2024-03-01 | Buy |
| Alfa Aesar | 087681 | Iodic acid, ACS, 99.5% | 7782-68-5 | 250g | $316 | 2024-03-01 | Buy |
| Alfa Aesar | A11925 | Iodic acid, 99% | 7782-68-5 | 50g | $77 | 2024-03-01 | Buy |
Iodic acid Chemical Properties,Uses,Production
Physical Properties
White stable crystalline solid; rhombohedral crystals; occurs in two forms: the normal HIO3, and pyroiodic acid HI3O8.
Uses
Aqueous solutions of iodic acid serve as strong oxidizing agents. The acid also is used in redox titrations.
Preparation
Iodic acid may be prepared by the reaction of sulfuric acid with barium iodate. The solution is filtered to remove barium sulfate and then crystallized to obtain iodic acid:
Ba(IO3)2 + H2SO4 → BaSO4 + 2HIO3
It also may be produced by oxidation of iodine with concentrated nitric acid:
3I2 + 10HNO3 → 6HIO3 + 10NO + 2H2O
Also, iodic acid may be obtained by oxidation of iodine with chlorine in dilute acidic solutions:
I2 + 5Cl2 + 6H2O → 2HIO3 + 10HCl
Another method of preparation involves oxidation of iodine with hydrogen peroxide:
I2 + 5H2O2 → 2HIO3 + 4H2O
It also may be prepared by treating hypoiodous acid with a base:
3HIO + 2OH¯ → HIO3 + 2H2O + I¯
Hypoiodous acid may be obtained by alkaline hydrolysis of iodine at pH 12:
I2 + H2O → HIO + H+ + I¯
Iodic acid dehydrates to iodine pentaoxide when heated at 180°C:
2HIO3 → I2H5 + H2O
Iodic acid is a relatively weak monoprotic acid, the Ka value at 25°C is 1.6 x 10–1. Several species have been detected in concentrated aqueous solutions, which include IO3¯, H+, HIO3, (HIO3)2 and (HIO3)3. Its solution turns blue litmus red and then bleaches the litmus paper because of its strong oxidizing properties.
When heated with potassium iodate, potassium hydrogen iodate is formed:
HIO3 + KIO3 → KH(IO3)2
An aqueous solution of iodic acid is a strong oxidizing agent. It liberates iodine from iodides:
IO3¯ + 5I¯ + 6H+ → 3I2 + 3H2O or,
HIO3 + 5HI → 3I2 + 3H2O
In an aqueous solution, iodic acid oxidizes hydrogen sulfide to sulfur:
2HIO3 + 5H2S → I2 + 6H2O + 5S
The solid iodic acid reacts vigorously with sulfur, phosphorus and other nonmetals.
Chemical Properties
colorless, rhomb crystal(s) or white, crystal(s) powder(s); darkens on exposure to light; it is a moderately strong acid; used in analytical chemistry and in medicine [HAW93] [MER06]
Physical properties
Iodic acid, HIO3, can be obtained as a white solid. It
dissolves in water very well, but it also exists in the
pure state, as opposed to chloric acid or bromic acid.
Iodic acid contains iodine in the oxidation state+5
and it is one of the most stable oxo-acids of the halogens
in its pure state. When iodic acid is carefully heated, it
dehydrates to iodine pentoxide. On subsequent heating,
the iodine pentoxide further decomposes, giving
a mixture of iodine, oxygen and lower oxides of iodine.
Iodic acid can be produced by oxidizing I2 with
chlorine in an aqueous solution. Iodic acid can be
used to synthesize sodium or potassium iodate salts
(which are used in salt as a source of iodine in the
human body.
Uses
A strong acid in analytical chemistry.
Uses
Iodic acid is used in analytical chemistry laboratories to standardize solutions of both weak and strong bases using methyl red or methyl orange as the indicator. It acts as a key starting material to synthesize sodium or potassium iodate, thereby increasing the iodine content of salt.
Definition
iodic acid: Any of various oxoacids of iodine, such as iodic(V) acid and iodic(VII) acid. When used without an oxidation state specifled, the term usually refers to iodic(V) acid (HIO3).
Preparation
Iodic acid may be prepared by the reaction of sulfuric acid with bariumiodate. The solution is filtered to remove barium sulfate and then crystallizedto obtain iodic acid:
Ba(IO3)2 + H2SO4 → BaSO4 + 2HIO3
It also may be produced by oxidation of iodine with concentrated nitric acid:
3I2 + 10HNO3 → 6HIO3 + 10NO + 2H2O
Also, iodic acid may be obtained by oxidation of iodine with chlorine in diluteacidic solutions:
I2 + 5Cl2 + 6H2O → 2HIO3 + 10HCl
Another method of preparation involves oxidation of iodine with hydrogenperoxide:
I2 + 5H2O2 → 2HIO3 + 4H2O
It also may be prepared by treating hypoiodous acid with a base:
3HIO + 2OH¯ → HIO3 + 2H2O + I¯
Hypoiodous acid may be obtained by alkaline hydrolysis of iodine at pH 12:
I2 + H2O → HIO + H+ + I¯
Iodic acid dehydrates to iodine pentaoxide when heated at 180°C:
2HIO3 → I2H5 + H2O
Iodic acid is a relatively weak monoprotic acid, the Ka value at 25°C is 1.6x10-1. Several species have been detected in concentrated aqueous solutions,which include IO3-, H+, HIO3, (HIO3)2 and (HIO3)3. Its solution turns blue lit-mus red and then bleaches the litmus paper because of its strong oxidizingproperties.
When heated with potassium iodate, potassium hydrogen iodate is formed:
HIO3 + KIO3 → KH(IO3)2
An aqueous solution of iodic acid is a strong oxidizing agent. It liberates iodine from iodides:
IO3¯ + 5I¯ + 6H+ → 3I2 + 3H2O or, HIO3 + 5HI → 3I2 + 3H2O
In an aqueous solution, iodic acid oxidizes hydrogen sulfide to sulfur:
2HIO3 + 5H2S → I2 + 6H2O + 5S
The solid iodic acid reacts vigorously with sulfur, phosphorus and other non-metals.
General Description
Iodic acid is the hydrated form of I2O5. Reaction of iodic acid with hydrogen iodide has been described by electrolytic dissociation theory. Combustion of mixture of chromic, iodic, sulfuric and phosphoric acids has been proposed. Its Raman spectra have been recorded. Vibrational assignment of IO3- has been evaluated.
Hazard
Toxic by ingestion, strong irritant to eyes and skin.
Purification Methods
Dissolve iodic acid in the minimum volume of hot dilute HNO3, filter and evaporate in a vacuum desiccator until crystals are formed. Collect the crystals and wash them with a little cold H2O and dry them in air in the dark. It is soluble in H2O: 269g/100mL at 20o and 295g/100mL at 40o. It is soluble in dilute EtOH and darkens on exposure to light. It is converted to HIO3.I2O5 on heating at 70o, but at 220o complete conversion to HIO3 occurs. [Lamb et al. J Am Chem Soc 42 1636 1920, Bray & Caulkins J Am Chem Soc 53 44 1931.]
Iodic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29811 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17365 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34553 | 58 |
View Lastest Price from Iodic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-19 | Iodic acid
7782-68-5
|
US $30.00 / KG | 1KG | 99% | 20 Tons | Wuhan wingroup Pharmaceutical Co., Ltd | |
 |
2023-01-31 | Iodic acid
7782-68-5
|
US $100.00 / kg | 1kg | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2020-01-13 | Iodic acid
782-68-5
|
US $1.00 / KG | 1KG | 99% | 100KG | Career Henan Chemical Co |
-

- Iodic acid
7782-68-5
- US $30.00 / KG
- 99%
- Wuhan wingroup Pharmaceutical Co., Ltd
-

- Iodic acid
7782-68-5
- US $100.00 / kg
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Iodic acid
782-68-5
- US $1.00 / KG
- 99%
- Career Henan Chemical Co