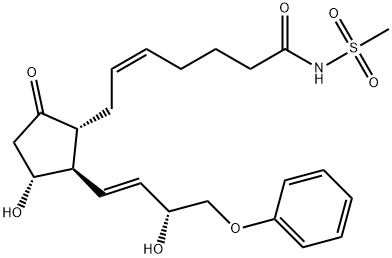
Sulprostone
- CAS No.
- 60325-46-4
- Chemical Name:
- Sulprostone
- Synonyms
- sch286;SHB-286;Nalador;ZK-57671;CP-34089;CP-34086;SULPROSTONE;Sulprostone USP/EP/BP;Sulprostone >=95% (HPLC), oil;Sulprostone in 5mg/ml Methyl Acetate
- CBNumber:
- CB3224098
- Molecular Formula:
- C23H31NO7S
- Molecular Weight:
- 465.56
- MDL Number:
- MFCD00216056
- MOL File:
- 60325-46-4.mol
- MSDS File:
- SDS
| Melting point | 79.25°C |
|---|---|
| Density | 1.1918 (rough estimate) |
| refractive index | 1.5650 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: >5 mg/mL |
| pka | 4.63±0.40(Predicted) |
| form | solid |
| color | white to off-white |
| FDA UNII | 501Q5EQ1GM |
| ATC code | G02AD05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H319-H335-H360 | |||||||||
| Precautionary statements | P201-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 60-36/37/38 | |||||||||
| Safety Statements | 53-22-36/37-45 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MJ8810000 | |||||||||
| Toxicity | LD50 intraperitoneal in mouse: 18mg/kg | |||||||||
| NFPA 704 |
|
Sulprostone price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S8692 | Sulprostone ≥95% (HPLC), oil | 60325-46-4 | 1mg | $223 | 2024-03-01 | Buy |
| Sigma-Aldrich | S8692 | Sulprostone ≥95% (HPLC), oil | 60325-46-4 | 5mg | $783 | 2024-03-01 | Buy |
| Cayman Chemical | 14765 | Sulprostone ≥98% | 60325-46-4 | 500μg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 14765 | Sulprostone ≥98% | 60325-46-4 | 1mg | $107 | 2024-03-01 | Buy |
| Cayman Chemical | 14765 | Sulprostone ≥98% | 60325-46-4 | 5mg | $437 | 2024-03-01 | Buy |
Sulprostone Chemical Properties,Uses,Production
Description
Sulprostone is a metabolism resistant synthetic analog of PGE2. It is a selective agonist for EP3 receptors with a Ki value of 0.35 nM at the human recombinant EP3-
Originator
Nalador,Schering,W. Germany,1981
Uses
antimalarial activity
Uses
Sulprostone is a selective agonist of the PGE2 receptor.
Uses
Human chondrocytes3 and mouse adrenal chromaffin cells4 were treated with sulprostone to study the biological effects of PGE2.
Definition
ChEBI: Sulprostone is a prostanoid.
Manufacturing Process
9α-Hydroxy-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-
13-ω-tetranorprostadienoicacid: To a solution of 1.6 g (3.6 mmols) (4-
carbohydroxy-n-butyl)triphenylphosphonium bromide in a dry nitrogen
atmosphere in 6.0 ml dry dimethyl sulfoxide was added 3.24 ml (6.5 mmols)
of a 2.0 M solution of sodium methylsulfinylmethide in dimethyl sulfoxide. To
this red ylide solution was added dropwise a solution of 613 mg (1.29 mmols)
2-[5α-hydroxy-3α-(tetrahydropyran-2-yloxy)-2α-(3α-tetrahydropyran-2-yloxy-
4-phenoxytrans-1-buten-1-yl)cyclopent-1α-yl] acetaldehyde, γ-hemiacetal in
5.0 ml dry dimethyl sulfoxide over a period of 20 minutes.
After an additional 2 hours stirring at room temperature, the reaction mixture
was poured onto ice water. The basic aqueous solution was washed twice with
ethyl acetate (20 ml) and acidified to pH 3 with 10% aqueous hydrochloric
acid.
The acidic solution was extracted with ethyl acetate (3 x 20 ml) and the
combined organic extracts washed once with water (10 ml), dried (MgSO4)
and evaporated to a solid residue. This solid residue was triturated with ethyl
acetate and the filtrate concentrated. Yield: 754 mg of 9α-hydroxy-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-
ωtetranorprostadienoic acid was collected.
9-Oxo-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-ω-
tetranorprostadienoicacid: To a solution cooled to -10°C under nitrogen of 754
mg (1.3 mmols) 9α-hydroxy-11α,15α-bis-(tetrahydropyran-2-yloxy)-16-
phenoxy-cis-5-trans-13-ω-tetranorprostadienoic acid in 13 ml reagent grade
acetone was added dropwise to 0.56 ml (1.41 mmols) of Jones' reagent
(chromic anhydride). After 20 minutes at -10°C, 0.260 ml 2-propanol was
added and the reaction mixture was allowed to stir an additional 5 minutes at
which time it was combined with 75 ml ethyl acetate, washed with water (3 x
10 ml), dried (MgSO4)and concentrated to give 752 mg of 9-oxo-11α,15α-bis-
(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-ω-tetranorprostadienoic
acid, which was chromatographed on silica gel using ethyl acetate as eluent to
afford 505 mg of pure intermediate.
N-Methanesulfonyl-9-oxo-11α,15α-dihydroxy-5-cis-13-trans-16-phenoxy-ω-
tetranorprostadienamide:To 1.0 mmols of 9-oxo-11α,15α-bis-
(tetrahydropyran-2-yloxy)-16-phenoxy-cis-5-trans-13-ω-tetranorprostadienoic
acid in 40 ml THF is added 2 ml triethylamine. After 15 minutes of stirring at
room temperature 10.0 ml of 0.1 M methanesulfonylisocyanate in THF is
added. After a further 1 hour of stirring, the reaction mixture is neutralized
with acetic acid and the solvent removed by evaporation (in vacuo). The
resultant residue is taken up in methylene chlorine and washed successively
with water and sodium bicarbonate to yield, after drying and solvent
evaporation, N-methanesulfonyl-9-oxo-11α,15α-bis-(tetrahydropyran-2-yloxy)-
16-phenoxy-cis-5-trans-13-ω-tetranorprostadienamide. This intermediate is
then hydrolyzed overnight with acetic acid/water and purified by column
chromatography to give the desired N-methanesulfonyl-9-oxo-11α,15α-
dihydroxy-5-cis-13-trans-16-phenoxy-ω-tetranorprostadienamide.
Therapeutic Function
Contraceptive
World Health Organization (WHO)
Sultopride, a neuroleptic indicated for the treatment of acute and chronic psychoses, was introduced on the market in 1976. In the early 1990s, its use was associated with cardiac arrhythmias, some of which were fatal. This led the regulatory authority in France to take restrictive action on the product. Sultopride continues to be marketed in several other countries.
Biochem/physiol Actions
Sulprostone is an analog of prostaglandin E2 (PGE2)1 and antagonizes vasopressin-induced antidiuretic responses in cells from rat renal inner medullae by a mechanism that involves activation of Rho.2
storage
Store at -20°C
Sulprostone Preparation Products And Raw materials
Sulprostone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15354 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52925 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Xi'an ZB Biotech Co.,Ltd | sales03@xazbbio.com | CHINA | 719 | 58 |