Vorinostat
- CAS No.
- 149647-78-9
- Chemical Name:
- Vorinostat
- Synonyms
- Suberoylanilide hydroxamic acid;N1-hydroxy-N8-phenyloctanediaMide;SAHA cpd;Vornostat;vorinosta;Vorinostat (SAHA, MK0683);suberoylaMide hydroxaMic acid;MK0683;CS-1949;FuLi, he
- CBNumber:
- CB3506806
- Molecular Formula:
- C14H20N2O3
- Molecular Weight:
- 264.32
- MDL Number:
- MFCD00945317
- MOL File:
- 149647-78-9.mol
- MSDS File:
- SDS
| Melting point | 161-162°C |
|---|---|
| Density | 1.2 |
| RTECS | RG8835000 |
| storage temp. | -20°C |
| solubility | DMSO: ≥15mg/mL |
| form | powder |
| pka | 9.48±0.20(Predicted) |
| color | white to tan |
| Merck | 14,10034 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 6 months. |
| InChIKey | WAEXFXRVDQXREF-UHFFFAOYSA-N |
| CAS DataBase Reference | 149647-78-9(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | suberoylanilide hydroxamic acid; vorinostat |
| FDA UNII | 58IFB293JI |
| NCI Drug Dictionary | vorinostat |
| ATC code | L01XH01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H341-H360 | |||||||||
| Precautionary statements | P201-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 61-68 | |||||||||
| Safety Statements | 53-36/37-45 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29280000 | |||||||||
| NFPA 704 |
|
Vorinostat price More Price(54)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0061 | SAHA ≥98% (HPLC) | 149647-78-9 | 5mg | $95.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0061 | SAHA ≥98% (HPLC) | 149647-78-9 | 25mg | $388 | 2024-03-01 | Buy |
| TCI Chemical | H1388 | N-Hydroxy-N'-phenyloctanediamide >98.0%(HPLC)(N) | 149647-78-9 | 200mg | $318 | 2024-03-01 | Buy |
| Alfa Aesar | H37305 | Vorinostat 98% | 149647-78-9 | 250mg | $117 | 2021-12-16 | Buy |
| Alfa Aesar | H37305 | Vorinostat 98% | 149647-78-9 | 1g | $269 | 2021-12-16 | Buy |
Vorinostat Chemical Properties,Uses,Production
Antitumor drugs
Vorinostat is a novel, molecularly targeted antineoplastic agent that causes cell cycle arrest and/or apoptosis by inhibiting histone deacetylase (HDAC). It is the first HDAC inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphoma (CTCL) with significant skin involvement that is still progressing, resistant or relapsing after two systemic regimens.
On October 6, 2006, the US Food and Drug Administration (FDA) have approved vorinostat capsules (vorinostat) for the treatment of skin cancer drugs. The drug is the first novel type of anti-cancer drugs of histone deacetylase inhibitor developed by the United States Merck for the treatment of skin T cell lymphoma (CTCL). FDA has approved it for the treatment of metastatic skin T-cell lymphoma which is unable to be cured or even worsened or gets recurrent cases. A large number of experimental studies and clinical results have shown that vorinostat has a excellent efficacy on a variety of tumors and have significant synergies when combined with other oncology drugs. The current treatment of other tumors is still undergoing in-depth study; these results show that vorinostat has a broad market prospects.
Vorinostat has low toxicity with the evidence of its safety and efficacy being supported by two clinical trials, including 107 patients with CTCL who had gotten relapsed after receiving other drugs. According to the standard analysis of improvement in the grade of skin lesion, 30% of patients treated with Zolinza get symptoms improved, with the average efficacy duration of 168 days. The most common serious adverse events were pulmonary embolism, dehydration, deep venous thrombosis and anemia. Common adverse reactions are gastrointestinal symptoms (including diarrhea, nausea and loss of appetite, vomiting and constipation); fatigue, chills and taste disorders. Animal experiments showed that pregnant women should be banned of using the drug.
Preparation
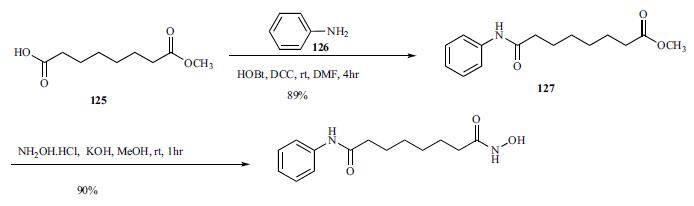
Suberic acid can undergo the intramolecular dehydration into suberic anhydride under the action of the acetic anhydride. The suberic anhydride, together with aniline can have ring-opening amidation in ethyl acetate at 0 °C to generate suberic acid monoanilide, followed by methanol esterification and hydroxylamine amine aminolysis to obtain the anti-tumor drug in vorinostat with the total yield of about 65%.
"Chinese Journal of Pharmaceutical Industry" 2009, Volume 40, No. 7, pages 481-483
Anti-cancer drug Vorinostat was able to clear latent HIV virus
Researchers from the University of North Carolina at Chapel Hill have published a groundbreaking research paper in the July 25, 2012 issue of Nature to confirm that a deacetylase inhibitor drug – vorinostat that can be used to treat certain types of lymphoma-being capable of clearing out the patient's latent HIV virus in vivo.
The researchers have conducted a series of experiments to evaluate the potential of this drug to activate and destroy latent HIV viruses. Initially, laboratory experiments for measuring the level of active HIV in CD4 + T cells showed that vorinostat can take off the camouflage of latent HIV viruses in these cells. Then, eight male patients who still kept medically stable HIV infection after antiretroviral therapy, took vorinostat, and then were tested their active HIV levels in the body and compared it to the levels they had before taking the drug.
The researchers found that HIV-RNA levels in CD4 + T cells increased by an average of 4.5-fold in those patients who receiving vorinostat, confirming that the HIV virus was disguised. This is the first published study confirming that deacetylase inhibitors have the potential to break down latency in latent virus libraries. The study provides convincing evidence that a new strategy may be used to directly attack and eradicate latent HIV infection. However, getting rid of the latent nature of HIV is only the first step in curing HIV infection.
Description
Vorinostat is the first drug in a new class of anti-cancer agents that inhibit histone deacetylases (HDAC). It was launched as an oral treatment for cutaneous manifestations in patients with cutaneous T-cell lymphoma (CTCL) who have progressive, persistent, or recurrent disease on or following two systemic therapies. HDACs are enzymes that catalyze the removal of the acetyl modification on lysine residues of proteins, including the core nucleosomal histones. Together with their counterpart histone acetyltransferases (HATs), HDACs regulate the acetylation level of the histones, which plays an important role in the regulation of chromatin plasticity and gene transcription. Hypoacetylation of histones is associated with a condensed chromatin structure resulting in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of transcription. In some cancer cells, there is an overexpression of HDACs, resulting in hypoacetylation of histones. Inhibitors of HDAC are thought to transcriptionally reactivate dormant tumor-suppressor genes by allowing for the accumulation of acetyl groups on histones and an open chromatin structure. Vorinostat inhibits the enzymatic activity of HDAC1, HDAC2, HDAC3, and HDAC6 at nanomolar concentrations (IC50 <86 nM). In vitro, it induces growth arrest, differentiation or apoptosis in a variety of tumor cells. In addition, vorinostat inhibits tumor growth in animal models bearing solid tumors, including breast, prostate, lung and gastric cancers, as well as hematologic malignancies such as multiple myeloma and leukemias.
Chemical Properties
White Crystalline Solid
Originator
Columbia University (US)
Uses
A potent HDAC inhibitor; also causes cell cycle arrest at G1
Uses
antineoplastic, histone deacetylase inhibitor
Uses
A potent, selective, cell permeable histone deacetylase inhibitor (HDAC). Displays anti-angiogenic activity by interfering with VEGF signaling in human umbilical vein endothelial cells (HUVECs). Induces differentiation in uman breast cancer cells.
Uses
Vorinostat, a histone deacetylase (HDAC) inhibitor from Merck, was approved for the treatment of cutaneous T-cell lymphoma (CTCL), a type of non-Hodgkin’s lymphoma. Vorinostat was shown to inhibit HDAC1, HDAC2, HDAC3 and HDAC6 at nanomolar concentrations. HDAC inhibitors are potent differentiating agents toward a variety of neoplasms, including leukemia and breast and prostate cancers.
Uses
Suberoylanilide Hydroxamic Acid is a potent, selective, cell permeable histone deacetylase inhibitor (HDAC). Suberoylanilide Hydroxamic Acid displays anti-angiogenic activity by interfering with VEGF signaling in human umbilical vein endothelial cells (HUVECs). Suberoylanilide Hydroxamic Acid induces differentiation in uman breast cancer cells.
Definition
ChEBI: A dicarboxylic acid diamide comprising suberic (octanedioic) acid coupled to aniline and hydroxylamine. A histone deacetylase inhibitor, it is marketed under the name Zolinza for the treatment of cutaneous T cell lymphoma (CTCL).
brand name
Zolinza
General Description
Histones are proteins around which DNA is wound in the process of packing DNA into the nucleus. They also havea role in regulating the transcription of genes, and this iscontrolled by the covalent modifications acetylation, phosphorylation,and methylation to which they are subject.
Vorinostat fits the basic pharmacophore for the HDACis, which consists of a hydrophobic cap regionconnected to a zinc coordinating functionality by a hydrophobiclinker.The hydroxamic acid functionality iscapable of bidendate binding to zinc present in the enzymeand is a major factor in the overall binding of the compound.The compound inhibits HDAC1, 2, 3, and 6 classes of thisenzyme with nanomolar (<86 nM) IC50 values.
The agent is given orally and is available in 100-mg capsulesfor the treatment of cutaneous T-cell lymphoma. Thebioavailability is 43%, and the agent is 71% bound toplasma proteins. Extensive metabolism of the agent occursto give the O-glucuronide of the hydroxamic acid functionand 4-anilino-4-oxobutanoic acid with minimal involvementof isozymes of CYP. The metabolites, both of whichare inactive, are eliminated in the urine and the drug has aterminal elimination half-life of 2 hours. The most commonlyreported adverse effects are fatigue, diarrhea, andnausea.
Biochem/physiol Actions
SAHA or Vorinostat facilitates the transcription of genes that result in apoptosis, differentiation and growth arrest. It has been observed to give beneficial results in lymphoma but not in solid tumors.
Synthesis
Commercially available monomethyl ester 125 was reacted with aniline in the presence of DCC and HOBt in DMF to give amide 127 in 89% yield.
storage
-20°C
References
1) Vrana et al. (1999), Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun and p21CIP1, but independent of p53; Oncogene, 18 7016 2) Butler et al. (2002), The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin; Proc. Natl. Acad. Sci. USA, 99 11700 3) Tang et al. (2012), Sorafenib and HDAC inhibitors synergize to kill CNS tumor cells, 13 567
Vorinostat Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12841 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 | admin@hbdangtong.com | China | 1000 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 997 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
View Lastest Price from Vorinostat manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-01 | Vorinostat
149647-78-9
|
US $100.00-75.00 / kg | 1kg | 99% | 5000Ton | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-29 | Vorinostat
149647-78-9
|
US $0.00 / KG | 1KG | 98.5%min | 10kgs | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Vorinostat
149647-78-9
|
US $44.00-86.00 / mg | 99.93% | 10g | TargetMol Chemicals Inc. |
-

- Vorinostat
149647-78-9
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Vorinostat
149647-78-9
- US $0.00 / KG
- 98.5%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Vorinostat
149647-78-9
- US $44.00-86.00 / mg
- 99.93%
- TargetMol Chemicals Inc.
149647-78-9(Vorinostat)Related Search:
1of4





