Xanthan gum
- CAS No.
- 11138-66-2
- Chemical Name:
- Xanthan gum
- Synonyms
- XANTHAN;GUM XANTHAN;Xanthan Gum powder;GLUCOMANNAN MAYO;Xanthane gum;Konjac extract;XANTHAM;Tarazine;Keltrol F;Xanthan g
- CBNumber:
- CB3735028
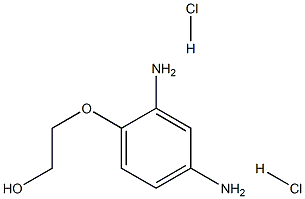
- Molecular Formula:
- C8H14Cl2N2O2
- Molecular Weight:
- 241.11496
- MDL Number:
- MFCD00131256
- MOL File:
- 11138-66-2.mol
- MSDS File:
- SDS
| Melting point | 64.43 °C |
|---|---|
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Soluble in water giving a highly viscous solution, practically insoluble in organic solvents. |
| form | Solid |
| color | Off-White to Pale Yellow |
| Odor | sl. organic odor, tasteless |
| biological source | Xanthomonas campestris |
| Viscosity | 25-70mPa.s1% in H2O(25°C) |
| Merck | 14,10057 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C8H12N2O2.2ClH/c9-6-1-2-8(7(10)5-6)12-4-3-11;;/h1-2,5,11H,3-4,9-10H2;2*1H |
| InChIKey | VXYWXJXCQSDNHX-UHFFFAOYSA-N |
| SMILES | C(O)COC1=CC=C(N)C=C1N.[H]Cl.[H]Cl |
| LogP | 3.926 (est) |
| FDA 21 CFR | 172.695; 573.1010; 310.545; 558.311 |
| Substances Added to Food (formerly EAFUS) | XANTHAN GUM |
| CAS DataBase Reference | 11138-66-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | TTV12P4NEE |
| Pesticides Freedom of Information Act (FOIA) | Xanthan gum |
| EPA Substance Registry System | Xanthan gum (11138-66-2) |
| Cosmetics Info | Xanthan Gum |
| UNSPSC Code | 12352201 |
| NACRES | NA.25 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H315 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
| Safety Statements | 24/25 |
| WGK Germany | 1 |
| HS Code | 32139000 |
Xanthan gum price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 43708 | Xanthan gum from Xanthomonas campestris | 11138-66-2 | 1kg | $585 | 2024-03-01 | Buy |
| Sigma-Aldrich | 42663 | Xanthan gum from Xanthomonas campestris 4,500,000 | 11138-66-2 | 100mg | $287 | 2023-06-20 | Buy |
| TCI Chemical | X0048 | Xanthan Gum | 11138-66-2 | 25g | $48 | 2024-03-01 | Buy |
| TCI Chemical | X0048 | Xanthan Gum | 11138-66-2 | 100g | $75 | 2024-03-01 | Buy |
| Sigma-Aldrich | G1253 | Xanthan gum from Xanthomonas campestris | 11138-66-2 | 100g | $94.2 | 2024-03-01 | Buy |
Xanthan gum Chemical Properties,Uses,Production
Description
Xanthan Gum is a long chain polysaccharide, which is made by mixing fermented sugars (glucose, mannose, and glucuronic acid) with a certain kind of bacteria. It is mainly used to thicken and stabilize emulsions, foams, and suspensions.
Xanthan gum is widely used as a food additive to control the rheological properties of a wide range of food products. In manufacturing, xanthan gum is used as a thickening and stabilizing agent in toothpastes and medicines. It is used to make medicine for lowering blood sugar and total cholesterol in people with diabetes. It is used as a laxative. Xanthan gum is sometimes used as a saliva substitute in people with dry mouth (Sjogren's syndrome).
Occurrence, Isolation
Xanthan gum, the extracellular polysaccharide from Xanthomonas campestris and some related microorganisms, is produced on a nutritive medium containing glucose, NH4Cl, a mixture of amino acids, and minerals. The polysaccharide is recovered from the medium by isopropanol precipitation in the presence of KCl.
analysis of bacterial polysaccharide
Xanthan gum is a high-molecular-weight polysaccharide gum produced by a pureculture fermentation of a carbohydrate with the gram-negative bacteria Xanthomonas campestris. This natural polysaccharide is widely used in the food industry and to a lesser extent in the pharmaceutical industry. Xanthan gum is monographed in the USP28/NF and in the PhEur. It is soluble in hot and cold water, as well as being stable under acidic and alkaline conditions (pH 5–13).
The primary structure of xanthan gum contains D-glucose and D-mannose as the dominant hexose units, along with D-glucuronic acid. The trisaccharide side chain consists of two D-mannose residues and one D-glucuronic acid residue occurring as mixed K+, Na+, and Ca++ salts. Through the association of xanthan molecules, it is thought that a quaternary structure arises through the charged trisaccharide side chains. Xanthan gum is a water-soluble polymer. Neither USP nor PhEur provides an HPLC method for determination of identity or purity.
References
[1] http://www.webmd.com
[2] https://en.wikipedia.org/wiki/Xanthan_gum
Chemical Properties
viscosity of 1% solution 1,200-1,600 mPas
Chemical Properties
Xanthan gum occurs as a cream- or white-colored, odorless, freeflowing, fine powder.
Uses
xanthan gum (corn starch gum) serves as a texturizer, carrier agent, and gelling agent in cosmetic preparations. It also stabilizes and thickens formulations. This gum is produced through a fermentation of carbohydrate and Xanthomonas campestris.
Uses
Xanthan Gum is a gum obtained by microbial fermentation from the xanthomonas campestris organism. it is very stable to viscosity change over varying temperatures, ph, and salt concentrations. it is also very pseudoplastic which results in a decrease in viscosity with increasing shear. it reacts synergistically with guar gum and tara gum to provide an increase in viscosity and with carob gum to provide an increase in viscosity or gel formation. it is used in salad dressings, sauces, desserts, baked goods, and beverages at 0.05–0.50%.
Uses
In foods, pharmaceuticals, and cosmetics as stabilizer and thickening agent. For rheology control in water-based systems. In oil and gas drilling and completion fluids.
Production Methods
Xanthan gum is a polysaccharide produced by a pure-culture aerobic fermentation of a carbohydrate with Xanthomonas campestris. The polysaccharide is then purified by recovery with propan-2-ol, dried, and milled.
brand name
Rhodigel (Vanderbilt).
General Description
As xanthan is a polysaccharide used in many applications such as a food additive, enzyme substrate or rheology modifier, it is useful to have a xanthan standard with a clearly defined narrow molecular weight distribution. Xanthan is produced by fermentation from Xanthomonas campestris.
Pharmaceutical Applications
Xanthan gum is widely used in oral and topical pharmaceutical
formulations, cosmetics, and foods as a suspending and stabilizing
agent. It is also used as a thickening and emulsifying agent. It is
nontoxic, compatible with most other pharmaceutical ingredients,
and has good stability and viscosity properties over a wide pH and
temperature range. Xanthan gum gels show
pseudoplastic behavior, the shear thinning being directly proportional
to the shear rate. The viscosity returns to normal immediately
on release of shear stress.
Xanthan gum has been used as a suspending agent for
conventional, dry and sustained-release suspensions. When
xanthan gum is mixed with certain inorganic suspending agents,
such as magnesium aluminum silicate, or organic gums, synergistic
rheological effects occur. In general, mixtures of xanthan gum and
magnesium aluminum silicate in ratios between 1 : 2 and 1 : 9
produce the optimum properties. Similarly, optimum synergistic
effects are obtained with xanthan gum : guar gum ratios between
3 : 7 and 1 : 9.
Although primarily used as a suspending agent, xanthan gum
has also been used to prepare sustained-release matrix tablets.
Controlled-release tablets of diltiazem hydrochloride prepared
using xanthan gum have been reported to sustain the drug release
in a predictable manner, and the drug release profiles of these tablets
were not affected by pH and agitation rate. Xanthan gum has
also been used to produce directly compressed matrices that display
a high degree of swelling due to water uptake, and a small amount
of erosion due to polymer relaxation. It has also been used in
combination with chitosan, guar gum, galactomannan, and sodium alginate to prepare sustained-release matrix
tablets. Xanthan gum has been used as a binder, and in
combination with Konjac glucomannan is used as an excipient
for controlled colonic drug delivery. Xanthan gum with boswellia
(3 : 1) and guar gum (10 : 20) have shown the best release
profiles for the colon-specific compression coated systems of 5-
fluorouracil for the treatment of colorectal cancer. Xanthan gum
has also been used with guar gum for the development of a floating
drug delivery system.It has also has derivatized to sodium
carboxymethyl xanthan gum and crosslinked with aluminum ions
to prepare microparticles, as a carrier for protein delivery.
Xanthan gum has been incorporated in an ophthalmic liquid
dosage form, which interacts with mucin, thereby helping in the
prolonged retention of the dosage form in the precorneal area.
When added to liquid ophthalmics, xanthan gum delays the release
of active substances, increasing the therapeutic activity of the
pharmaceutical formulations.
Xanthan gum can be used to increase the bioadhesive strength in
vaginal formulations. Xanthan gum alone or with carbopol
974P has been used as a mucoadhesive controlled-release excipient
for buccal drug delivery. Modified xanthan films have been
used as a matrix system for transdermal delivery of atenolol.
Xanthan gum has also been used as a gelling agent for topical
formulations incorporating solid lipid nanoparticles of vitamin
A or microemulsion of ibuprofen. A combined polymer system consisting of xanthan gum, carboxy methylcellulose and a
polyvinyl pyrolidone backboned polymer has been used for
relieving the symptoms of xerostomia. Xanthan gum can also
be used as an excipient for spray-drying and freeze-drying processes
for better results. It has been successfully used alone or in
combination with agar for microbial culture media.
Xanthan gum is also used as a hydrocolloid in the food industry,
and in cosmetics it has been used as a thickening agent in
shampoo. Polyphosphate with xanthum gum in soft drinks is
suggested to be effective at reducing erosion of enamel
Safety Profile
When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Xanthan gum is widely used in oral and topical pharmaceutical
formulations, cosmetics, and food products, and is generally
regarded as nontoxic and nonirritant at the levels employed as a
pharmaceutical excipient.
The estimated acceptable daily intake for xanthan gum has been
set by the WHO at up to 10 mg/kg body-weight.
No eye or skin irritation has been observed in rabbits and no skin
allergy has been observed in guinea pigs following skin exposure.
No adverse effects were observed in long term feeding studies with
rats (up to 1000 mg/kg/day) and dogs (up to 1000 mg/kg/day). No
adverse effects were observed in a three-generation reproduction
study with rats (up to 500 mg/kg/day).
LD50 (dog, oral): >20 g/kg
LD50 (rat, oral): >45 g/kg
LD50 (mouse, oral): >1 g/kg
LD50 (mouse, IP): >50 mg/kg
LD50 (mouse, IV): 100–250 mg/kg
storage
Xanthan gum is a stable material. Aqueous solutions are stable over
a wide pH range (pH 3–12), although they demonstrate maximum
stability at pH 4–10 and temperatures of 10–60°C. Xanthan gum
solutions of less than 1% w/v concentration may be adversely
affected by higher than ambient temperatures: for example,
viscosity is reduced. Xanthan gum provides the same thickening,
stabilizing, and suspending properties during long-term storage at
elevated temperatures as it does at ambient conditions. In addition,
it ensures excellent freeze–thaw stability. Solutions are also stable in
the presence of enzymes, salts, acids, and bases. Vanzan NF-ST is
especially designed for use in systems containing high salt
concentrations as it dissolves directly in salt solutions, and its viscosity is relatively unaffected by high salt levels as compared with
general purpose grades.
The bulk material should be stored in a well-closed container in a
cool, dry place.
Incompatibilities
Xanthan gum is an anionic material and is not usually compatible
with cationic surfactants, polymers, or preservatives, as precipitation
occurs. Anionic and amphoteric surfactants at concentrations
above 15% w/v cause precipitation of xanthan gum from a
solution.
Under highly alkaline conditions, polyvalent metal ions such as
calcium cause gelation or precipitation; this may be inhibited by the
addition of a glucoheptonate sequestrant. The presence of low levels
of borates (<300 ppm) can also cause gelation. This may be avoided
by increasing the boron ion concentration or by lowering the pH of
a formulation to less than pH 5. The addition of ethylene glycol,
sorbitol, or mannitol may also prevent this gelation.
Xanthan gum is compatible with most synthetic and natural
viscosity-increasing agents, many strong mineral acids, and up to
30% inorganic salts. If it is to be combined with cellulose
derivatives, then xanthan gum free of cellulase should be used to
prevent depolymerization of the cellulose derivative. Xanthan gum
solutions are stable in the presence of up to 60% water-miscible
organic solvents such as acetone, methanol, ethanol, or propan-2-
ol. However, above this concentration precipitation or gelation
occurs.
The viscosity of xanthan gum solutions is considerably
increased, or gelation occurs, in the presence of some materials
such as ceratonia, guar gum, and magnesium aluminum silicate.
This effect is most pronounced in deionized water and is reduced by
the presence of salt. This interaction may be desirable in some
instances and can be exploited to reduce the amount of xanthan
gum used in a formulation.
Xanthan gum is incompatible with oxidizing agents, some tablet
film-coatings, carboxymethylcellulose sodium, dried aluminum
hydroxide gel, and some active ingredients such as
amitriptyline, tamoxifen, and verapamil.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral solutions, suspensions, and tablets; rectal and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Xanthan gum Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 | L18532138899@163.com | China | 939 | 58 |
| Shijiazhuang Tongyang Import and Export Co., LTD | 18031361688; | admin@sjztongyang.com | China | 977 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10244 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 +86-18829239519 | sales06@tgybio.com | China | 898 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
| Maanshan Tiantai Biotechnology Co., Ltd. | +86-0555-8889890 +86-17356584060 | GM@tiantaibio-tech.com | China | 72 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3007 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +8617736087130 | catherine@yjchem.com.cn | China | 994 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615350851019 | admin@86-ss.com | China | 1001 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
Related articles
- Is Xanthan gum safe for daily use as a food additive?
- Xanthan gum is an FDA-approved food additive (stabilizer and emulsifier) without any restrictions. It is soluble in cold water....
- Jan 16,2025
- The difference between Xanthan Gum and Guar Gum
- When it comes to gluten-free baking, you may come across the question of whether to use Xanthan Gum or Guar Gum.
- Mar 4,2024
- Health benefits and Safety of Xanthan gum
- Xanthan gum is a polysaccharide with many industrial uses, including as a common food additive. It is an effective thickening ....
- Jul 25,2022
View Lastest Price from Xanthan gum manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-11 | Xanthan gum
11138-66-2
|
US $0.00-0.00 / Kg/Drum | 1KG | Viscosity≥0.6Pa.s | 20 Tons | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-11 | Xanthan gum
11138-66-2
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2025-04-11 | Xanthan gum
11138-66-2
|
US $1.00 / kg | 1kg | 99% | 1000mt/year | Jinan Finer Chemical Co., Ltd |
-

- Xanthan gum
11138-66-2
- US $0.00-0.00 / Kg/Drum
- Viscosity≥0.6Pa.s
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Xanthan gum
11138-66-2
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd
-

- Xanthan gum
11138-66-2
- US $1.00 / kg
- 99%
- Jinan Finer Chemical Co., Ltd