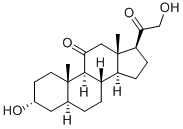
Alphadolone
- CAS No.
- 14107-37-0
- Chemical Name:
- Alphadolone
- Synonyms
- alfadolone;ALPHADOLONE;Aalfadolone;5α-PREGNAN-3α, 21-DIOL-11, 20-DIONE;3a,21-Dihydroxy-5a-pregnane-11,20-dione;(3α,5α)-3,21-Dihydroxypregnane-11,20-dione;(3α,5α)-3,21-Dihydroxypregnane-11,20-dione;Pregnane-11,20-dione, 3,21-dihydroxy-, (3α,5α)-;3a,21-DihydroxyChemicalbook-5a-pregnane-11,20-dione;Pregnane-11,20-dione, 3,21-dihydroxy-, (3.alpha.,5.alpha.)-
- CBNumber:
- CB3918218
- Molecular Formula:
- C21H32O4
- Molecular Weight:
- 348.48
- MDL Number:
- MFCD00200115
- MOL File:
- 14107-37-0.mol
| Melting point | >145°C (dec.) |
|---|---|
| Boiling point | 508.5±50.0 °C(Predicted) |
| Density | 1.175±0.06 g/cm3(Predicted) |
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.95±0.10(Predicted) |
| color | White to Off-White |
| FDA UNII | OE1C96974E |
Alphadolone price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | A575510 | Alphadolone | 14107-37-0 | 5mg | $215 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD17323 | 3a,21-Dihydroxy-5a-pregnane-11,20-dione | 14107-37-0 | 2mg | $270 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD17323 | 3a,21-Dihydroxy-5a-pregnane-11,20-dione | 14107-37-0 | 5mg | $425 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | SHG0004905 | 3ALPHA,21-DIHYDROXY-5ALPHA-PREGNANE-11,20-DIONE 95.00% | 14107-37-0 | 5MG | $496.76 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD17323 | 3a,21-Dihydroxy-5a-pregnane-11,20-dione | 14107-37-0 | 10mg | $650 | 2021-12-16 | Buy |
Alphadolone Chemical Properties,Uses,Production
Originator
Alphadolone ,RiboTargets Ltd.
Uses
Alphadolone or Alfadolone (INN) is a neuroactive steroid with hypnotic effects. Alphadolone is one of the components of Althesin.
Manufacturing Process
A solution of 3β-acetoxy-5α-pregn-16-ene-11,20-dione (Chamberlin et al.,
J.Amer. Chem Soc., 1951, 73, 2396) (25.7 g) in dioxan (Analar, 500 ml) was
treated with potassium hydroxide (10 g) and water 250 ml and the mixture
allowed to stand at room temperature for 1 h. After a further 1 h at 40°C the
mixture was diluted with water and the product filtered off. The crude material
was dissolved in chloroform and filtered through a column of grade III neutral
alumina (100 g). The material obtained was crystallized from acetonepetroleum
to give pure 3β-hydroxy-5α-pregn-16-ene-11,20-dione (17.65 g,
77.5%) as small plates, melting point 217.5°C.
A solution of 3β-hydroxy-5α-pregn-16-ene-11,20-dione (39.6 g) in dry
pyridine (165 ml) was treated with toluene-p-sulfonyl chloride (43.9 g) to give
the toluene sulfonate (56.7 g), melting point 147-151°C. A portion (10.7 g) of
this material was crystallized from ethyl acetate-petroleum to give the pure
3β-toluene-p-sulfonyloxy-5α-pregn-16-ene-11,20-dione (9.2 g) as plates,
melting point 154°-155°C.
2 Methods of producing of 3α-hydroxy-5α-pregn-16-ene-11,20-dione from 3β-
toluene-p-sulfonyloxy-5α-pregn-16-ene-11,20-dione:
1. A solution of 3β-toluene-p-sulfonyloxy-5α-pregn-16-ene-11,20-dione (19.1
g) in N,N-dimethylformamide (160 ml) and water (16.0 ml) was treated with
potassium acetate (29.2 g) and the mixture heated at 115°C for 2.5 h. The
solvents were removed in vacuo and residue partitioned between chloroform
and water. The chloroform extract was washed with water, dried and
evaporated. The residue was taken up in methanol (500 ml) and solution
flushed with nitrogen. Potassium hydroxide (17 g) in water (70 ml) was added
and the solution refluxed for 1 h. Glacial acetic acid was added to bring the pH
to about 6 and most of the methanol evaporated in vacuo. Dilution with water
gave a gummy precipitate which was extracted into chloroform to give the
crude product. This material was extracted with ether and the residue boiled
with benzene. The insoluble material was crystallized from chloroformpetroleum
to give 3α-hydroxy-5α-pregn-16-ene-11,20-dione (3.28 g) as large prisms, melting point 243°-244°C.
2. A mixture of the 3β-toluene-p-sulfonyloxy-5α-pregn-16-ene-11,20-dione
(60 g; 0.124 mole) in N,N-dimethylformamide (350 ml) and potassium
acetate (92 g, 0.94 mole) in water (935 ml) was stirred at 115°C for 4 h. The
brown solution was cooled and most of the N,N-dimethylformamide removed
by evaporation at 50°C and 4 mm to give a brown solid mass. Another run
with to sylate (58 g, 0.12 mole), potassium acetate (90 g, 0.91 mole), N,Ndimethylformamide
(350 ml) and water (35 ml) was carried out as described
above. The combined aqueous fractions were extracted with chloroform (3 x
100 ml) and dried over magnesium sulfate. The chloroform was removed in
vacuo and residual N,N-dimethylformamide was evaporated at 50°C and 4
mm to give the crude 3α-acetate-5α-pregn-16-ene-11,20-dione (92 g) as a
brown solid.
A solution of 3α-acetate-5α-pregn-16-ene-11,20-dione (92 g) in dioxin (1000
ml) was mixed with a solution of potassium hydroxide (45 g, 0.8 mole) in
water (500 ml) to give a two-phase system. A homogeneous solution was
obtained by the addition of dioxin (440 ml) and water (625 ml). Nitrogen was
bubbled through the solution which was heated at 50°C for 2 h. The port
colored solution was treated with glacial acetic acid (40 ml) to bring the pH
about 7 and two thirds of the solvent was removed by distillation in vacuo
(water pump). Water (3 L) was added to the resultant mixture (which had
already begun to crystallize) and the precipitated solid was filtered off, washed
with water and dried over phosphorus pentoxide to give the crude 3α-
hydroxy-5α-pregn-16-ene-11,20-dione (73.9 g).
Producing of 3α,21-dihydroxy-5α-pregnane-11,20-dione from 3α-hydroxy-5α-
pregn-16-ene-11,20-dione:
A solution of 3α-hydroxy-5α-pregn-16-ene-11,20-dione (200 mg) in freshly
distilled tetrahydrofuran (8 ml) with 5% palladium on carbon (100 mg) was
hydrogenated till hydrogen uptake ceased. The mixture was filtered through a
pad of kieselguhr and the tetrahydrofuran removed in vacuo to give 3α-
hydroxy-5α-pregnane-11,20-dione (196 mg), melting point 171°-172°C.
Boron trifluoride etherate (37.9 ml) was added to a stirred solution of 3α-
hydroxy-5α-pregnane-11,20-dione (6.64 g, 20 mmol) and lead tetraacetate
(10.1 g, 22 mmol) in dry benzene (280 ml) and methanol (15.1 ml) at room
temperature. After 2 h the mixture was poured into water (2 L) and extracted
with ether (1 L). The combined ether extracts were washed successively with
sodium bicarbonate solution and water, dried over magnesium sulfate, and
concentrated in vacuo to give a white crystalline mass. Four recrystallizations
from acetone-petroleum (b.p. 40°-60°C) gave 21-acetoxy-3α-hydroxy-5α-
pregnane-11,20-dione as fine needles (4.22 g, 54%), melting point 172°-
173°C.
The 3α,21-dihydroxy-5α-pregnane-11,20-dione is conveniently prepared by
the deacylation of 21-acetoxy-3α-hydroxy-5α-pregnane-11,20-dione under
basic conditions, for example, in the presence of potassium or sodium
hydrogen carbonate, conveniently in the presence of a solvent e.g. methanol,
ethanol or tetrahydrofuran, reesterifying the resultant product.
Therapeutic Function
Anesthetic
Clinical Use
Althesin is an effective intravenous anesthetic for short surgical procedures or for induction of lengthy anesthesia . Doses of 0.050 – 0.075mLper kg of body weight produce anesthesia in adults for 5 – 20 min and analgesia to surgical stimuli for 2 – 5 min. Althesin causes cardiodepressive side effects. Although it is used in countries in which French pharmaceuticals dominate, it is hardly used in Germany.
Alphadolone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22883 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| Hunan Yuanye Pharmaceutical Co., Ltd. | +8615857615745 | 2675013845@qq.com | China | 87 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38632 | 58 | |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Wuhan Jingkang en Biomedical Technology Co., Ltd | +8613720134139 | orders@jknbiochem.com | China | 5221 | 58 |
| TargetMol Chemicals Inc. | +8613564774135 | zijue.cai@tsbiochem.com | United States | 19885 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 94657 | 76 |
View Lastest Price from Alphadolone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-24 | Alfadolone
14107-37-0
|
US $2500.00-1980.00 / mg | 10g | TargetMol Chemicals Inc. | |||
 |
2024-04-02 | Alphadolone
14107-37-0
|
US $0.00 / kg | 1kg | 99% | 20tons | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2022-05-13 | Alphadolone
14107-37-0
|
US $1.10 / g | 1g | 99.0% Min | 100 Tons | Dideu Industries Group Limited |
-

- Alfadolone
14107-37-0
- US $2500.00-1980.00 / mg
- TargetMol Chemicals Inc.
-

- Alphadolone
14107-37-0
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Alphadolone
14107-37-0
- US $1.10 / g
- 99.0% Min
- Dideu Industries Group Limited