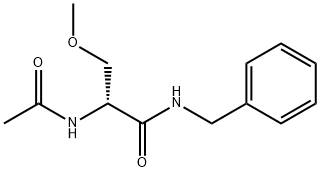
Lacosamide
- CAS No.
- 175481-36-4
- Chemical Name:
- Lacosamide
- Synonyms
- ViMpat;Erlosamide;Lacosamide API;(R)-2-Acetamido-N-benzyl-3-methoxypropanamide;(2R)-2-acetamido-N-benzyl-3-methoxy-propanamide;SPM 927;ACOSAMIDE;LACOSAMIDE;ADD 243037;Lacoxamide
- CBNumber:
- CB41011740
- Molecular Formula:
- C13H18N2O3
- Molecular Weight:
- 250.29
- MDL Number:
- MFCD08272557
- MOL File:
- 175481-36-4.mol
| Melting point | 141-143?C |
|---|---|
| alpha | D23 +16.0° (c = 1 in CH3OH) |
| Boiling point | 536.4±50.0 °C(Predicted) |
| Density | 1.120±0.06 g/cm3(Predicted) |
| Flash point | 2℃ |
| storage temp. | Refrigerator |
| solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; Ethanol: 20 mg/ml; PBS (pH 7.2): 2 mg/ml |
| form | A crystalline solid |
| pka | 14.19±0.46(Predicted) |
| InChI | InChI=1S/C13H18N2O3/c1-10(16)15-12(9-18-2)13(17)14-8-11-6-4-3-5-7-11/h3-7,12H,8-9H2,1-2H3,(H,14,17)(H,15,16)/t12-/m1/s1 |
| InChIKey | VPPJLAIAVCUEMN-GFCCVEGCSA-N |
| SMILES | C(NCC1=CC=CC=C1)(=O)[C@H](NC(C)=O)COC |
| CAS DataBase Reference | 175481-36-4 |
| FDA UNII | 563KS2PQY5 |
| NCI Drug Dictionary | lacosamide |
| ATC code | N03AX18 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H312+H332-H319 | |||||||||
| Precautionary statements | P210-P280-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xn | |||||||||
| Risk Statements | 11-20/21/22-36 | |||||||||
| Safety Statements | 16-26-36/37 | |||||||||
| RIDADR | UN 1648 3 / PGII | |||||||||
| WGK Germany | 2 | |||||||||
| HS Code | 2924296000 | |||||||||
| NFPA 704 |
|
Lacosamide price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | L-029 | Lacosamide solution 1.0?mg/mL in acetonitrile, ampule of 1?mL, certified reference material, Cerilliant? | 175481-36-4 | 1mL | $202 | 2024-03-01 | Buy |
| Cayman Chemical | 10012592 | Lacosamide ≥98% | 175481-36-4 | 5mg | $49 | 2024-03-01 | Buy |
| Cayman Chemical | 10012592 | Lacosamide ≥98% | 175481-36-4 | 10mg | $87 | 2024-03-01 | Buy |
| Cayman Chemical | 10012592 | Lacosamide ≥98% | 175481-36-4 | 25mg | $152 | 2024-03-01 | Buy |
| Cayman Chemical | 10012592 | Lacosamide ≥98% | 175481-36-4 | 50mg | $278 | 2024-03-01 | Buy |
Lacosamide Chemical Properties,Uses,Production
Description
Lacosamide (LCM), (SPM 927, (R)-2-acetamido-N-benzyl-3-methoxypropionamide, previously referred to as harkoseride or ADD 234037) is a member of a series of functionalized amino acids that were specifically synthesized as anticonvulsant or antiepileptic drug. It reduces the spread of seizure activity in the brain. Lacosamide appears to have a dual mode of action: selective enhancement of sodium channel inactivation and modulation of collapsin response mediator protein-2. Compared to novel antiepileptic drugs, lacosamide has broader and higher efficacy, better tolerability, and improved pharmacokinetic properties. Lacosamide is in phase III clinical development for adjunctive treatment of patients with uncontrolled partial-onset seizures, and for monotherapy of patients with painful diabetic neuropathy. It is absorbed rapidly and completely after oral administration. Lacosamide has an elimination half-life of approximately 13 hours and a low potential for drug interactions. Additionally, lacosamide exhibits linear, dose-proportional pharmacokinetics with low intra- and interpatient variability.
Lacosamide (licensed in 2008) is a third- generation AED known with the proprietary brand name of Vimpat® (UCB Pharma, Slough) in the UK and USA.
Generic formulation
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (incl. generic products):
- It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
Indications
Epilepsy: Adjunctive treatment of focal seizures with or without secondary generalization.
Dose titration
- Epilepsy— adjunctive therapy: 50 mg bd for 7 days, then increased by 50 mg bd every 7 days; usual maintenance 100 mg bd (max. 200 mg bd).
- Epilepsy— adjunctive therapy (loading dose regimen when it is necessary to rapidly attain therapeutic plasma concentrations, under close medical supervision): 200 mg bd for 1 day, followed by maintenance dose of 100 mg bd after 1 day, then increased if needed by 50 mg bd every 7 days (max. 200 mg bd).
Uses
Lacosamide is an anticonvulsant and analgesic compound used for the treatment of partial-onset seizures and neuropathic pain. It can also used for the treatment of status epilepticus. Its analgesic effect is mediated by its inhibitory effect on sodium channels, which leads to neural membrane depolarization. Its interaction with the collapsin-response mediator protein 2 (CRMP-2 alias DRP-2) may also facilitate the above process, although the detailed mechanism remains to be elucidated.
Side effects
Lacosamide was generally well tolerated in adult patients with partial-onset seizures.[11] The side-effects most commonly leading to discontinuation were dizziness, ataxia, diplopia (double vision), nystagmus, nausea, vertigo and drowsiness. These adverse reactions were observed in at least 10% of patients.[9] Less common side-effects include tremors, blurred vision, vomiting and headache.
Cautions
- Patients with conduction problems (contraindicated in patients with second- or third- degree A– V block).
- Patients with severe cardiac disease.
- Patients at risk of PR- interval prolongation.
- Elderly patients.
Interactions
With AEDs
Concomitant treatment with other AEDs known to be enzyme inducers (such as carbamazepine, phenobarbital, phenytoin) decreases the overall systemic exposure of lacosamide by 25%.
With other drugs
Nil.
With alcohol/food
- Although no pharmacokinetic data on the interaction of lacosamide with alcohol are available, a pharmacodynamic effect cannot be excluded.
- There are no specific foods that must be excluded from diet when taking lamotrigine.
Special populations
Hepatic impairment
- Titrate with caution in mild- to- moderate impairment if co- existing renal impairment.
- Caution in severe impairment.
Renal impairment
- Loading dose regimen can be considered in mild- to- moderate impairment (titrate above 200 mg with caution).
- Titrate with caution in severe impairment (max. 250 mg daily).
Pregnancy
- There are no adequate data from the use of lacosamide in pregnant women and the potential risk for humans is unknown.
- Lacosamide should not be used during pregnancy unless clearly necessary (if the benefit to the mother clearly outweighs the potential risk to the foetus). If a woman decides to become pregnant, the use of lacosamide should be carefully re- evaluated. In case of treatment with lacosamide, the dose should be monitored carefully during pregnancy and after birth, and adjustments made on a clinical basis.
- Lacosamide has been found to be present in milk in animal studies and it is recommended that it should be avoided during breastfeeding
Behavioural and cognitive effects in patients with epilepsy
For this third- generation agent, clinical experience is still limited and little is known about its positive and negative psychotropic properties and their implications for the management of behavioural symptoms in patients with epilepsy. There are initial reports of depression, irritability and agitation, and psychotic symptoms. Reports of cognitive effects (mainly affecting attention and memory) are rare and usually not severe.
Psychiatric use
Lacosamide has no indications for the treatment of psychiatric disorders. There is insufficient experience with lacosamide to draw any conclusion regarding its psychotropic profile.
References
[1] B. K. Beyreuther, J. Freitag, C. Heers, N. Krebsfänger, U. Scharfenecker, T. Stöhr (2007) Lacosamide: a review of preclinical properties, CNS Drug Reviews, 13, 21-42
[2] Beyreuther, Bettina K., et al. "Lacosamide: A Review of Preclinical Properties." Cns Drug Reviews 13.1(2007):21.
[3]Chung, S, et al. "Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial." Epilepsia 51.6(2010):958–967.
[4]Kellinghaus, C, et al. "Intravenous lacosamide for treatment of status epilepticus." Acta Neurologica Scandinavica 123.2(2011):137–141.
Description
Lacosamide selectively enhances sodium channel slow inactivation without affecting fast inactivation. It is effective in multiple rodent models of seizure activity. The neuroprotective effects of lacosamide are also attributed to its ability to modulate collapsin response mediator protein 2 (CRMP-2), a member of the semaphorin signal transduction pathway. Formulations containing lacosamide have been used as an adjunctive or monotherapy for focal-onset seizures but, at higher doses, have a low potential for abuse. Lacosamide is regulated as a Schedule V compound in the United States.
Description
Although epilepsy is a neurological disorder with varying etiology and severity, the common feature is unprovoked, recurring seizures. Whether classified as generalized, involving both cerebral hemispheres, or partial with only localized portions of brain participation at onset, effective treatment relies on accurate assessment of syndrome type to optimally decrease the frequency, duration, and severity of seizures. The latest weapon against partial onset epilepsy is lacosamide, formerly known as harkoseride and erlosamide. The data also indicate that lacosamide binds to collapsing response mediator protein 2 (CRMP2); CRMP2 is involved in neuronal differentiation, control of axonal outgrowth, and possibly epileptogenesis. Furthermore, lacosamide is heralded as having a dual mode of action as it has also displayed efficacy against diabetic neuropathy, possibly as a result of stabilization of neuronal hyperexcitability. Currently,lacosamide is approved as adjunctive treatment of partial onset seizures in patients 17 years or older and is in development as a monotherapy for epilepsy and for neuropathic pain.
Chemical Properties
White to Off-White Solid
Originator
Harris FRC (United States)
Uses
A potent anticonvulsant.
Definition
ChEBI: Lacosamide is a N-acyl-amino acid.
brand name
Vimpat
Synthesis
The most common adverse events were diplopia, headache, dizziness, and nausea. As typical with AEDs, lacosamide may increase the risk of suicidal thoughts or behavior. Patients should, therefore, be monitored for the emergence or worsening of depression. Caution should also be exercised in patients with known conduction problems or severe cardiac disease (myocardial ischemia or heart failure) since dose-dependent prolongations in PR interval have been observed in clinical studies.
Lacosamide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Hope Pharmaceutical Co., Ltd. | +86-010-67886402 +8613611125266 | market@hopelife.cn | China | 71 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Nanjing Gold Pharmaceutical Technology Co. Ltd. | 025-84209270 15906146951 | CHINA | 115 | 55 | |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9214 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
Related articles
- lacosamide vs carbamazepine
- Lacosamide (LCM) is a widely used third-generation antiseizure drug (ASD) approved for treating focal epilepsy.
- Sep 11,2024
- What is LACOSAMIDE?Uses,Pharmacodynamics,Mechanism of action,Side effects
- Lacosamide is a functionalized amino acid that has activity in the maximal electroshock seizure test, and is indicated for the....
- Aug 24,2020
View Lastest Price from Lacosamide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Lacosamide
175481-36-4
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-21 | Lacosamide
175481-36-4
|
US $0.00 / KG | 1KG | 98.0% | 100kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-10-25 | LACOSAMIDE
175481-36-4
|
US $30.00-1.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. |
-

- Lacosamide
175481-36-4
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Lacosamide
175481-36-4
- US $0.00 / KG
- 98.0%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- LACOSAMIDE
175481-36-4
- US $30.00-1.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
175481-36-4(Lacosamide)Related Search:
1of4