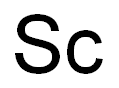SCANDIUM
- CAS No.
- 7440-20-2
- Chemical Name:
- SCANDIUM
- Synonyms
- SCANDIUM;SC000205;SC000200;SC000211;SC000212;SC000210;Scandium-45;Scandium lump;scandium atom;SCANDIUM METAL
- CBNumber:
- CB4115641
- Molecular Formula:
- Sc
Lewis structure

- Molecular Weight:
- 44.96
- MDL Number:
- MFCD00016323
- MOL File:
- 7440-20-2.mol
| Melting point | 1540 °C (lit.) |
|---|---|
| Boiling point | 2836 °C (lit.) |
| Density | 2.99 g/mL at 25 °C (lit.) |
| solubility | Slowly dissolve in dilute acids. |
| form | powder |
| color | Silver-gray |
| Specific Gravity | 3 |
| Resistivity | 50.5 μΩ-cm, 0°C |
| Water Solubility | Reacts with water. |
| Sensitive | air sensitive |
| Merck | 13,8468 |
| Exposure limits |
ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| InChIKey | SIXSYDAISGFNSX-UHFFFAOYSA-N |
| CAS DataBase Reference | 7440-20-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | YUJ4U1EW7R |
| EPA Substance Registry System | Scandium (7440-20-2) |
| Poissons Ratio | 0.279 |
|---|---|
| Shear Modulus | 29.1 GPa |
| Hardness, Vickers |
36, (0101) face 132, (0001) face |
| Bulk Modulus | 56.6 GPa |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H228 | |||||||||
| Precautionary statements | P210-P240-P241-P280-P370+P378 | |||||||||
| Hazard Codes | F,T,Xi | |||||||||
| Risk Statements | 5-11-34-23/24/25-36/37/38-36/38 | |||||||||
| Safety Statements | 26-28-36/37-7/9-36/37/39-16-43-45-27 | |||||||||
| RIDADR | UN 3089 4.1/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2805304000 | |||||||||
| NFPA 704 |
|
SCANDIUM price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 261262 | Scandium powder, 99.9% trace rare earth metals basis | 7440-20-2 | 250mg | $220 | 2023-06-20 | Buy |
| Sigma-Aldrich | 261262 | Scandium powder, 99.9% trace rare earth metals basis | 7440-20-2 | 1g | $642 | 2023-01-07 | Buy |
| Alfa Aesar | 035755 | Scandium plasma standard solution | 50mL | $184 | 2024-03-01 | Buy | |
| Alfa Aesar | 035755 | Scandium plasma standard solution | 100mL | $280 | 2024-03-01 | Buy | |
| Alfa Aesar | 035755 | Scandium plasma standard solution | 500mL | $673 | 2024-03-01 | Buy |
SCANDIUM Chemical Properties,Uses,Production
General Description
Scandium, a transition metal with a silvery appearance, is often classified as a non-lanthanide rare earth element. It is widely used in various products, such as fluorescent lamps, baseball bats and bicycle frames. In industry, it is primarily alloyed to other metal compounds to produce high performance materials. Currently, the only known concentrated sources of scandium are thortveitite, euxenite and gadolinite, which are rare minerals from Scandinavia and Madagascar.
Uses
Scandium is mainly used in ceramics, lasers, phosphors and crystal. Scandium Oxide is suitable for the high index component of UV, AR and bandpass coatings due to its high index value, transparency, and layer hardness make high damage thresholds have been reported for combinations with Silicon Dioxide or Magnesium Fluoride for use in AR.
Scandium Metal is applied in various superalloys which show excellent metallic performance with their light gravaty. The main application of Scandium by weight is in Scandium-Aluminium alloys for minor aerospace industry components. They were used in the Russian military aircraft, specifically the MiG-21 and MiG-29. Some items of sports equipment, which rely on high performance materials, have been made with Scandium-Aluminium alloys. Employed in solid state synthesis of unusual clusters, Sc19Br28Z4, (Z=Mn, Fe, Ru or Os). These clusters are of interest for their structure and magnetic properties.
Chemical Properties
grey powder
Physical properties
Scandium is a soft, lightweight, silvery-white metal that does not tarnish in air, but overtime, it turns yellowish-pink. It resists corrosion. Scandium reacts vigorously with acids, butnot water. Scandium has some properties similar to the rare-earth elements. Although its position in group 3 places it at the head of the 17 elements of the lanthanide series of rare-earthmetals, scandium, as a metal, is not usually considered a rare-earth. Scandium’s melting pointis 1,541°C, its boiling point is 2836°C, and its density is 2.989 g/cm3.
Isotopes
There are 28 isotopes of scandium, ranging from scandium-36 to scandium-57.Scandium-45 is the only stable isotope and contains about 100% of the natural scandium found in the Earth’s crust. The remaining isotopes are radioactive with half-livesranging from nanoseconds to a few minutes to a few hours to a few days, and therefore,they are not found naturally in the Earth’s crust. The radioactive isotopes of scandiumare produced in nuclear reactors.
Origin of Name
From the Latin word Scandia, for “Scandinavia.
Occurrence
Although scandium is chemically similar to rare-earths, it no longer is considered to be oneof them. Scandium is the 42nd most abundant element found in the Earth’s crust, makingup about 0.0025% of the Earth’s crust. It is widely distributed at 5 ppm on the Earth. (It isabout as abundant as lithium, as listed in group 1.) Scandium is even more prevalent in thesun and several other stars than it is on Earth.Scandium is found in ores of wolframite in Norway and thortveitite in Madagascar. It isalso found in granite pegmatites and monazites. It is common in many of the ores where tinand tungsten are also found.
History
On the basis of the Periodic System, Mendeleev predicted the existence of ekaboron, which would have an atomic weight between 40 of calcium and 48 of titanium. Scandium was discovered by Nilson in 1878 in the minerals euxenite and gadolinite, which had not yet been found anywhere except in Scandinavia. By processing 10 kg of euxenite and other residues of rare-earth minerals, Nilson was able to prepare about 2 g of scandium oxide of high purity. Cleve later pointed out that Nilson’s scandium was identical with Mendeleev’s ekaboron. Scandium is apparently a much more abundant element in the sun and certain stars than here on Earth. It is about the 23rd most abundant element in the sun, compared to the 50th most abundant on Earth. It is widely distributed on Earth, occurring in very minute quantities in over 800 mineral species. The blue color of beryl (aquamarine variety) is said to be due to scandium. It occurs as a principal component in the rare mineral thortveitite, found in Scandinavia and Malagasy. It is also found in the residues remaining after the extraction of tungsten from Zinnwald wolframite, and in wiikite and bazzite. Most scandium is presently being recovered from thortveitite or is extracted as a by-product from uranium mill tailings. Metallic scandium was first prepared in 1937 by Fischer, Brunger, and Grieneisen, who electrolyzed a eutectic melt of potassium, lithium, and scandium chlorides at 700 to 800°C. Tungsten wire and a pool of molten zinc served as the electrodes in a graphite crucible. Pure scandium is now produced by reducing scandium fluoride with calcium metal. The production of the first pound of 99% pure scandium metal was announced in 1960. Scandium is a silver-white metal that develops a slightly yellowish or pinkish cast upon exposure to air. It is relatively soft, and resembles yttrium and the rare-earth metals more than it resembles aluminum or titanium. It is a very light metal and has a much higher melting point than aluminum, making it of interest to designers of spacecraft. Scandium is not attacked by a 1:1 mixture of conc. HNO3 and 48% HF. Scandium reacts rapidly with many acids. Twentythree isotopes and isomers of scandium are recognized. The metal is expensive, costing about $200/g with a purity of about 99.9%. About 20 kg of scandium (as Sc203) are now being used yearly in the U.S. to produce high-intensity lights, and the radioactive isotope 46Sc is used as a tracing agent in refinery crackers for crude oil, etc. Scandium iodide added to mercury vapor lamps produces a highly efficient light source resembling sunlight, which is important for indoor or night-time color TV. Little is yet known about the toxicity of scandium; therefore, it should be handled with care.
Characteristics
Scandium is the first element in the fourth period of the transition elements, which meansthat the number of protons in their nuclei increases across the period. As with all the transition elements, electrons in scandium are added to an incomplete inner shell rather than tothe outer valence shell as with most other elements. This characteristic of using electrons inan inner shell results in the number of valence electrons being similar for these transition elements although the transition elements may have different oxidation states. This is also whyall the transition elements exhibit similar chemical activity.
Uses
Scandium was not produced in any quantities until the late 1930s. Its light weight,resistance to corrosion, and high melting point made it especially useful in the aerospaceindustries. In the early 1940s contractors for the U.S. Air Force appropriated almost all of thescandium metal for use in the construction of military aircraft. The pure metal form is produced by the electrolysis of a salt of scandium, ScCl3. The metal has found some other uses in high-intensity lamps that produce a natural spectrum, making it useful for stadium lighting.It is also used in nickel alkaline storage batteries. Several compounds are used as catalysts tospeed up chemical reactions. The radioactive scandium-46 (with a half-life of 83.8 days) emitsbeta radiation. This property makes it useful as a radioactive tracer in the petroleum industryto monitor fractionation products.
Uses
It finds applications in the aerospace industry. Another use of Scandium is in aluminum-scandium alloys for sports equipment, such as bicycle frames, lacrosse sticks, and baseball bats.
Definition
scandium: Symbol Sc. A rare softsilvery metallic element belonging togroup 3 (formerly IIIA) of the periodictable; a.n. 21; r.a.m. 44.956; r.d.2.989 (alpha form), 3.19 (beta form);m.p. 1541°C; b.p. 2831°C. Scandiumoften occurs in lanthanoid ores,from which it can be separated on accountof the greater solubility of itsthiocyanate in ether. The only naturalisotope, which is not radioactive,is scandium–45, and there are nineradioactive isotopes, all with relativelyshort half-lives. Because of themetal’s high reactivity and high costno substantial uses have been foundfor either the metal or its compounds.Predicted in 1869 by DmitriMendeleev, and then called ekaboron,the oxide (called scandia) wasisolated by Lars Nilson (1840–99) in1879.
Definition
A lightweight silvery element belonging to the first transition series. It is found in minute amounts in over 800 minerals, often associated with lanthanoids. Scandium is used in high-intensity lights and in electronic devices. Symbol: Sc; m.p. 1541°C; b.p. 2831°C; r.d. 2.989 (0°C); p.n. 21; r.a.m. 44.955910.
Hazard
As with other metals, the transition metals and many of their compounds are toxic, andtheir powdered or gaseous forms should not be ingested or inhaled. In addition, all but one ofthe isotopes of scandium are radioactive and should be handled by experienced personnel.
Toxicity evaluation
Scandium is considered non-toxic, though extensive animal testing of scandium compounds has not been done.The median lethal dose (LD50) levels for scandium chloride for rats have been determined as 755 mg/kg for intraperitoneal and 4 g/kg for oral administration.Compounds of scandium should be handled as compounds of moderate toxicity.
SCANDIUM Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 2904 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| Henan Alfa Chemical Co., Ltd | China | 13225 | 58 | ||
| Alfa Chemistry | Info@alfa-chemistry.com | United States | 24072 | 58 | |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Mainchem Co., Ltd. | -- | sarah@mainchem.com | China | 6567 | 58 |
Related articles
- What is scandium used for?
- Scandium is mainly used for research purposes. It has, however, great potential as it has almost the same low density as alumi....
- May 29,2024
- Scandium: Importance, Effects on Microorganisms and Bioaccumulation
- Scandium's vital role in high-tech industries is hindered by mining challenges, yet its diverse impact on microorganisms holds....
- Apr 10,2024
- Scandium: distribution and applications
- Scandium's unique properties enhance performance, energy efficiency, and durability across aerospace, sports, electronics, and....
- Nov 13,2023