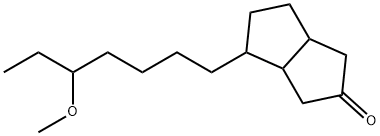
Cioteronel
- CAS No.
- 89672-11-7
- Chemical Name:
- Cioteronel
- Synonyms
- Cioteronel;Cioteronelum;Unii-1rth95874z;Cioteronelum [inn-latin];6-(5-Methoxyheptyl)bicyclo[3.3.0]octan-3-one;2(1H)-Pentalenone, hexahydro-4-(5-methoxyheptyl)-
- CBNumber:
- CB41178296
- Molecular Formula:
- C16H28O2
- Molecular Weight:
- 252.39
- MDL Number:
- MOL File:
- 89672-11-7.mol
| FDA UNII | 1RTH95874Z |
|---|
Cioteronel Chemical Properties,Uses,Production
Originator
Cyoctol,Squibb
Uses
Anti-androgen.
Definition
ChEBI: Cioteronel is a cyclic ketone.
Manufacturing Process
3-(5-Methoxyhept-1-yl)cyclopentene:
A three-neck, round-bottomed flask containing magnesium metal turnings
(7.2 g, 0.299 moles), is equipped with a Friedrich condenser and kept under a
nitrogen atmosphere. Tetrahydrofuran (300 ml) is added and the contents are
allowed to stir. A solution of 1-chloro-5-methoxyheptane (48.1 g, 0.292
moles) is added in small portions and refluxed. The mixture is allowed to stir
for 3 hours. The resultant dark yellow solution is cooled to -25°C, and the
condenser is removed and replaced with a dry ice addition funnel. A solution
of 3-chlorocyclopentene (29.9 g, 0.292 moles) is added over a period of one
hour. The viscous solution is poured into two liters of saturated ammonium
chloride, extracted with ether, and dried over anhydrous sodium sulfate.
Distillation yields 3-(5-methoxyhept-1-yl)cyclopentene (51.5 g, 0.262 moles)
as clear, colorless oil boiling at about 90°C/0.3 mm and 54°C/0.1 mm.
6,6-Dichloro-2-(5-methoxyhept-1-yl)bicyclo[3.2.0]heptan-7-one:
A 1,000 ml three-neck, round-bottomed flask, containing 3-(5-methoxyhept-
1-yl)cyclopentene (15.0 g, 0.076 moles) in 300 ml of hexane, is equipped
with a reflux condenser. Freshly distilled dichloroacetyl chloride (35.1 g, 0.240
moles) is added and the solution stirred and heated to reflux. Triethylamine
(25.2 g, 0.249 moles) in 200 ml hexane, is added dropwise to the refluxing
solution and the solution allowed to stir for 4 hours. The solvent is removed
and the residue distilled and chromatographically purified with silica gel,
leaving the 6,6-dichloro-2-(5-methoxyhept-1-yl)bicyclo[3.2.0]heptan-7-one
(17 g).
6,6-Dichloro-2-(5-methoxyhept-1-yl)bicyclo[3.3.0]octan-7-one:
6,6-Dichloro-2-(5-methoxyhept-1-yl)bicyclo[3.2.0]heptan-7-one (5 g), is
dissolved in 100 ml of ether and transferred to a 500 ml, round-bottomed
flask. An excess of diazomethane is generated in situ by reacting N-methyl-N?nitroso-p-toluene sulfonamide (60 g) with potassium hydroxide in ethanol.
The diazomethane is allowed to react for 50 min, after which time acetic acid
is added to destroy any remaining diazomethane. The solution is extracted
with ether and dried over anhydrous sodium sulfate and yields the crude 6,6-
dichloro-2-(5-methoxyhept-1-yl)bicyclo[3.3.0]octan-7-one as an orange oil.
2-(5-Methoxyhept-1-yl)bicyclo[3.3.0]octan-7-one:
6,6-Dichloro-2-(5-methoxyhept-1-yl)bicyclo[3.3.0]octan-7-one (45.9 g) is
added to a 100 ml, round-bottomed flask fitted with a condenser. Powdered
zinc metal (92 g) and glacial acetic acid (312 ml) are added to the flask and
1034 Cioteronel
the solution allowed to reflux for an hour. The solution is filtered to remove
the zinc and zinc chloride, formed in the reaction. The product is washed with
an aqueous sodium bicarbonate solution and extracted three times with ether.
The ether extracts are combined and dried over anhydrous sodium sulfate.
The resulting yellow oil is chromatographed on silica gel and eluted with 4:1
hexane:ether. The fractions are combined, and gave 2-(5-methoxyhept-1-
yl)bicyclo[3.3.0]octan-7-one as a clear, colorless oil.
Therapeutic Function
Antiandrogen
Cioteronel Preparation Products And Raw materials
Cioteronel Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Amatek Scientific Co. Ltd. | 0512-56316828 | info@amateksci.com | China | 28817 | 58 |
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.cn | China | 16377 | 58 |
| Supplier | Advantage |
|---|---|
| Amatek Scientific Co. Ltd. | 58 |
| TargetMol Chemicals Inc. | 58 |