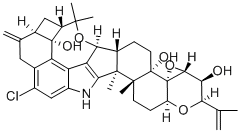
PENITREM A
- CAS No.
- 12627-35-9
- Chemical Name:
- PENITREM A
- Synonyms
- PENITREM A;TREMORTIN A;JDUWHZOLEDOQSR-JKPSMKLGSA-N;Penitrem A Nsc354845;PENITREM A, PENICILLIUM PAXILLI;Penitrem A from Penicillium paxilli;penitrem A from penicillium palitans;7,8-(Epoxymethano)-2H,6H-cyclobuta[5,6]benz[1,2-e]oxireno[4',4'a]-1-benzopyrano[5',6':6,7]indeno[1,2-b]indole-3,4b,7d(5H,7H)-triol, 12-chloro-3,3a,6a,8,9,9a,10,11,14,14b,14c,15,16,16a-tetradecahydro-14b,14c,17,17-tetramethyl-10-methylene-2-(1-methylethenyl)-, (2R,3S,3aR,4aS,4bS,6aR,7S,7dR,8R,9aR,14b...;2R,3S,3aR,4aS,4bS,6aR,7S,7dR,8R,9aR,14bS,14cR,16aS)-12-chloro-3,3a,6a,8,9,9a,10,11,14,14b,14c,15,16,16a-tetradecahydro-14b,14c,17,17-tetramethyl-10-methylene-2-(1-methylethenyl)-7,8-(epoxymethano)-2H,6H-cyclobuta[5,6]benz[1,2-e]oxireno[4',4'a]-1-benzopyrano[5',6':6,7]indeno[1,2-b]indole-3,4b,7d(5H,7H)-triol
- CBNumber:
- CB4155421
- Molecular Formula:
- C37H44ClNO6
- Molecular Weight:
- 634.2
- MDL Number:
- MFCD12547934
- MOL File:
- 12627-35-9.mol
- MSDS File:
- SDS
| Melting point | 238°C |
|---|---|
| Density | 1.0241 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 6 mg/ml) |
| form | White solid. |
| pka | 13.22±0.70(Predicted) |
| color | Amorphous solid |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| EWG's Food Scores | 1 |
| FDA UNII | 244AU85PR7 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300 | |||||||||
| Precautionary statements | P301+P330+P331+P310 | |||||||||
| Hazard Codes | T+,T | |||||||||
| Risk Statements | 26/27/28 | |||||||||
| Safety Statements | 36/37/39-45 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RY7535000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29349990 | |||||||||
| Toxicity | LD50 orl-mus: 10 mg/kg 41KEAL -,108,78 | |||||||||
| NFPA 704 |
|
PENITREM A price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P3053 | Penitrem A ≥95% (HPLC and TLC) | 12627-35-9 | 1mg | $340 | 2024-03-01 | Buy |
| Sigma-Aldrich | P3053 | Penitrem A ≥95% (HPLC and TLC) | 12627-35-9 | 5mg | $1220 | 2024-03-01 | Buy |
| Cayman Chemical | 11347 | Penitrem A ≥98% | 12627-35-9 | 1mg | $41 | 2024-03-01 | Buy |
| Cayman Chemical | 11347 | Penitrem A ≥98% | 12627-35-9 | 5mg | $152 | 2024-03-01 | Buy |
| Cayman Chemical | 11347 | Penitrem A ≥98% | 12627-35-9 | 10mg | $284 | 2024-03-01 | Buy |
PENITREM A Chemical Properties,Uses,Production
Description
Penitrem A (12627-35-9) is a fungal mycotoxin that acts as a selective, irreversible blocker of the smooth muscle high conductance Ca2+-activated K+?(BK, KCa1.1) channel (100% block at 10 nM).1?Displays brain neurotoxicity in rats along with dose-dependent convulsions and death.2?An important tool for studying the role of BK channels in vascular function which is effective in cellular, tissue and?in vivo?experiments.3?Inhibits BK channels in inside-out and cell-attached patches, whereas iberiotoxin (considered the gold standard BK channel blocker) does not.3?May be used to partially ablate Purkinje cells in immature rat cerebellum providing a model for neural stem cell transplantation studies.4
Chemical Properties
Solid
Occurrence
A series of closely related metabolites of Penicillium crustosum has recently been isolated and the components separated chromatographically. Penitrem A forms colourless crystals from EtOH.
Uses
Penitrem A is a tremorgenic mycotoxin isolated from Penicillium species. Penitrem A is a selective blocker of high-conductance Ca2+-activated potassium channels.
Uses
Penitrem A has been used as Ca2+-Activated K+ (BK) channel blocker to study its effect on BK current in bag cell neurons.
Definition
ChEBI: Penitrem A is an organooxygen compound and an organic heterotricyclic compound.
Biochem/physiol Actions
Penitrem A intoxication causes ataxia, polypnea, and sustained tremors and may lead to seizures and death. Penitrem A affects the central and peripheral nervous system. It impairs the GABAergic neurotransmission in the cerebellum.
Safety Profile
Poison by ingestion and intraperitoneal routes. An experimental teratogen. When heated to decomposition it emits very toxic fumes of Clí and NOx.
storage
-20°C
References
References/Citations:
PENITREM A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30239 | 58 |
| BOC Sciences | 16314854226; +16314854226 | inquiry@bocsci.com | United States | 19741 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 | |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51456 | 80 |
| Alta Scientific Co., Ltd. | 022-6537-8550 15522853686 | sales@altasci.com.cn | China | 4511 | 55 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8447 | 60 |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9806 | 58 |
View Lastest Price from PENITREM A manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-28 | Penitrem A
12627-35-9
|
US $695.00-98.00 / mg | 10g | TargetMol Chemicals Inc. |
-

- Penitrem A
12627-35-9
- US $695.00-98.00 / mg
- TargetMol Chemicals Inc.