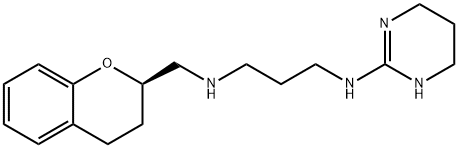
Alniditan
- CAS No.
- 152317-89-0
- Chemical Name:
- Alniditan
- Synonyms
- Alnitidan;1,3-Propanediamine, N1-[[(2R)-3,4-dihydro-2H-1-benzopyran-2-yl]methyl]-N3-(1,4,5,6-tetrahydro-2-pyrimidinyl)-
- CBNumber:
- CB43175523
- Molecular Formula:
- C17H26N4O
- Molecular Weight:
- 302.41
- MDL Number:
- MFCD00937785
- MOL File:
- 152317-89-0.mol
| storage temp. | Store at -20°C |
|---|---|
| solubility | Soluble in DMSO |
| FDA UNII | B57Z82EOGE |
Alniditan Chemical Properties,Uses,Production
Originator
Pasmigren ,Janssen-Cilag
Uses
Antimigraine.
Manufacturing Process
To a stirred and heated +80°C mixture of 3,4-dihydro-2H-1-benzopyran-2-
carboxylic acid and methylbenzene were added dropwise thionyl chloride
during a period of 85 min. Upon complete addition, stirring was continued for
2 h at 80°C. After cooling to room temperature, the reaction mixture was
evaporated. The residue was taken up in methylbenzene and the solvent was
evaporated again, yielding (R)-3,4-dihydro-2H-1-benzopyran-2-carbonyl
chloride.
A mixture of (R)-3,4-dihydro-2H-1-benzopyran-2-carbonyl chloride in N,Ndimethylacetamide
and 2,2'-oxybispropane was hydrogenated in the presence
of palladium-on-charcoal catalyst (10%) and a solution of thiophene in
methanol (4%). After the calculated amount of hydrogen was taken up, the
catalyst was filtered off and the filtrate was added to a mixture of
benzenemethanamine, potassium acetate and methanol. This mixture was
hydrogenated again in the presence of palladium-on-charcoal catalyst (10%)
and a solution of thiophene in methanol (4%). After the calculated amount of
hydrogen was taken up, the catalyst was filtered off and the filtrate was
evaporated. The residue was poured into water and the whole was basified
with NaOH (50%). The product was extracted with dichloromethane and the
extract was dried, filtered and evaporated. The residue was purified by column
chromatography (silica gel; CH2Cl2/CH3OH 95:5). The eluent of the desired
fraction was evaporated and the residue was converted into the hydrochloride
salt in 2-propanone by adding 2-propanol saturated with HCl. The salt was
filtered off and dried, yielding (R)-3,4-dihydro-N-(phenylmethyl)-2H-1-
benzopyran-2-methanamine.
A mixture of 28.0 g of (R)-3,4-dihydro-N-(phenylmethyl)-2H-1-benzopyran-2-
methanamine and 300 ml of methanol was hydrogenated in the presence of
2.0 g of palladium-on-charcoal catalyst (10%). After the calculated amount of
hydrogen was taken up, the catalyst was filtered off and the filtrate was
evaporated, yielding 18.2 g (100%) of (-)-(R)-3,4-dihydro-2H-1-benzopyran-
2-methanamine as crude residue.
A mixture of 18.0 g of (-)-(R)-3,4-dihydro-2H-1-benzopyran-2-methanamine,
60.0 g of 2-propenenitrile and 400 ml of ethanol was stirred for 4 h at reflux
temperature. The reaction mixture was evaporated and the residue was dried,
yielding 20.0 g (84%) of (-)-(R)-3-[[(3,4-dihydro-2H-1-benzopyran-2-
yl)methyl]amino]propanenitrile.
A mixture of 20.0 g (-)-(R)-3-[[(3,4-dihydro-2H-1-benzopyran-2-
yl)methyl]amino]propanenitrile and 300 ml of methanol was hydrogenated in
the presence of 5.0 g of Raney Nickel. After the calculated amount of
hydrogen was taken up, the catalyst was filtered off and the filtrate was
evaporated, yielding 21.0 g (100%) of (-)-(R)-N-[(3,4-dihydro-2H-1-
benzopyran-2-yl)methyl]-1,3-propanediamine as crude residue.
A mixture of (-)-(R)-N-[(3,4-dihydro-2H-1-benzopyran-2-yl)methyl]-1,3-
propanediamine, 2-chloropyrimidine, sodium carbonate and ethanol was
stirred for 4 h at reflux temperature. The reaction mixture was evaporated.
The residue was purified by column chromatography (silica gel; CHCl3/CH3OH
90:10). The eluent of the desired fraction was evaporated and the residue was
converted into the hydrochloride salt in 2-propanol. The salt was filtered off
and dried in vacuum, yielding (-)-(R)-N-[(3,4-dihydro-2H-1-benzopyran-2-
yl)methyl]-N'-(2-pyrimidinyl)-1,3-propanediamine dihydrochloride
hemihydrate.
A mixture of 3.6 g of (-)-(R)-N-[(3,4-dihydro-2H-1-benzopyran-2-yl)methyl]-
N'-(2-pyrimidinyl)-1,3-propanediamine dihydrochloride hemihydrate in 150 ml
of methanol and 20 ml of 2-propanol saturated with HCl was hydrogenated in
the presence of 1.5 g of palladium-on-charcoal catalyst (2%). After the
calculated amount of hydrogen was taken up, the catalyst was filtered off and
the filtrate was evaporated. The product was crystallized from acetonitrile,
filtered off and dried, yielding 2.7 g (74.0%) of (-)-(R)-N-[(3,4-dihydro-2H-1-
benzopyran-2-yl)methyl]-N'-(1,4,5,6-tetrahydro-2-pyrimidinyl)-1,3-
propanediamine dihydrochloride hemihydrate; melting point 200.2°C.
The base (-)-(R)-N-[(3,4-dihydro-2H-1-benzopyran-2-yl)methyl]-N'-(1,4,5,6-
tetrahydro-2-pyrimidinyl)-1,3-propanediamine may be obtained by treatment
of (-)-(R)-N-[(3,4-dihydro-2H-1-benzopyran-2-yl)methyl]-N'-(1,4,5,6-
tetrahydro-2-pyrimidinyl)-1,3-propanediamine dihydrochloride hemihydrate
with NaOH.
Therapeutic Function
Migraine therapy
in vitro
In vitro, alniditan exhibits little vasoconstrictive effects on the rat basilar artery, although at a very high concentration 1 mM, alniditan causes intensive constriction, most likely through a mechanism independent from 5-HT receptor activation. Alniditan is 10 times more potent than sumatriptan at the h5-HT1B receptor, and twice as potent at the h5-HT 1D receptor.
in vivo
The intraperitoneal administration of alniditan ED 50 =9 μg/kg and sumatriptan ED 50 =70 μg kg dose dependly reduces [ 125 I]-BSA extravasation in the rat meninges when done 30 min before stimulation. The estimated ED values for alniditan are 9 μg/kg in the absence and 190 μg/kg in the presence of GR 127935. Alniditan (3, 10, 30 and 100 μg/kg) produces a dose-dependent increase in the arteriovenous oxygen saturation difference, which seems to be attenuated in animals treated with GR127935. Alniditan dose-dependently decreases total carotid and arteriovenous anastomotic blood flow and concomitant conductance values; nutrient blood flow and conductance increase. Alniditan also produces significant increases in vascular conductance to the skin, ear, bone, salivary gland, fat, tongue, brain and dura mater; no changes are observed in the muscles and eyes.
Alniditan Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
Alniditan Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Fan De(Beijing) Biotechnology Co., Ltd. | 15911056312 | liming@bio-fount.com | China | 9730 | 58 |
| Beijing xinyanhui pharmaceutical research and development co., LTD | 13969155946 | 1461866103@qq.com | China | 16111 | 58 |
| cjbscvictory | 13348960310 13348960310 | 3003867561@qq.com | China | 10011 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 15801484223; | psaitong@jm-bio.com | China | 29778 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24017 | 58 |
| Nantong QuanYi Biotechnology Co., Ltd | 0513-66337626 18051384581 | sales@chemhifuture.com | China | 4455 | 58 |