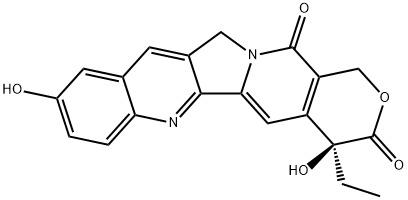
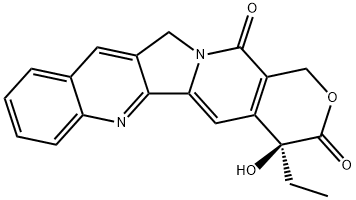
10-Hydroxycamptothecin
- CAS No.
- 19685-09-7
- Chemical Name:
- 10-Hydroxycamptothecin
- Synonyms
- SN38;HCPT;HYDROXYCAMPTOTHECIN;10-HCPT;(S)-10-HYDROXYCAMPTOTHECIN;Ampule;NSC 107124;HYDROCAMPTOTHECINE;hydroxy-camptotheci;Irinotecan USP RC A
- CBNumber:
- CB4445430
- Molecular Formula:
- C20H16N2O5
- Molecular Weight:
- 364.35
- MDL Number:
- MFCD00189425
- MOL File:
- 19685-09-7.mol
- MSDS File:
- SDS
| Melting point | 265-270°C |
|---|---|
| Boiling point | 820.7±65.0 °C(Predicted) |
| Density | 1.60 |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≥23.8 mg/mL in DMSO with gentle warming; insoluble in EtOH; insoluble in H2O |
| form | powder to crystal |
| pka | 8.93±0.40(Predicted) |
| color | White to Yellow to Orange |
| InChIKey | HAWSQZCWOQZXHI-FQEVSTJZSA-N |
| CAS DataBase Reference | 19685-09-7(CAS DataBase Reference) |
| FDA UNII | 9Z01632KRV |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H340 | |||||||||
| Precautionary statements | P202-P264-P270-P280-P301+P310-P405 | |||||||||
| Safety Statements | 24/25 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
10-Hydroxycamptothecin price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2491 | Irinotecan Related Compound A Pharmaceutical Secondary Standard; Certified Reference Material | 19685-09-7 | 25MG | $485 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1347610 | Irinotecan Related Compound A | 19685-09-7 | 10mg | $1490 | 2024-03-01 | Buy |
| TCI Chemical | H1463 | 10-Hydroxycamptothecin >98.0%(HPLC) | 19685-09-7 | 1g | $231 | 2024-03-01 | Buy |
| Cayman Chemical | 14635 | (S)-10-hydroxy-Camptothecin ≥98% | 19685-09-7 | 25mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 14635 | (S)-10-hydroxy-Camptothecin ≥98% | 19685-09-7 | 50mg | $107 | 2024-03-01 | Buy |
10-Hydroxycamptothecin Chemical Properties,Uses,Production
Description
DNA topoisomerases relax supercoiled DNA during replication, transcription, recombination, repair, and chromosome condensation. The relaxation of DNA supercoiling by topoisomerase I at single-
Chemical Properties
Yellow Solid
Uses
A Camptothecin derivative; a topoisomerase inhibitor for cancer therapy
Definition
ChEBI: 10-Hydroxycamptothecin is a pyranoindolizinoquinoline.
in vitro
10-Hydroxycamptothecin inhibited the growth of BT-20 and MDA-231 cells with IC50 of 34.3nM and 7.27nM, respectively, which was more potent than camptothecin (CPT) with IC50>500nM. 10-Hydroxycamptothecin potently induces the formation of the pBR322 plasmid DNA cleavage complex mediated by human topoisomerase I with an EC50 of 0.35 μM, more than 50-fold more potent than CPT with an EC50 of 18.85 μM. 10-Hydroxycamptothecin treatment caused dose-dependent growth inhibition of human microvascular endothelial cells (HMECs) with IC50 of 0.31 μM and significantly inhibited HMEC migration with IC50 of 0.63 μM. Treatment of HMEC cells with 10-Hydroxycamptothecin also inhibited microtubule formation in a dose-dependent manner with IC50 of 0.96 μM. 10-Hydroxycamptothecin (5-20 nM) significantly inhibits the differentiation of Colo205 cells, arrests the cell cycle in G2 phase, and induces apoptosis through a caspase-3-dependent pathway.
in vivo
In the CAM model, 10-Hydroxycamptothecin treatment inhibited angiogenesis in a concentration-dependent manner, with 95% inhibition at 25 nM, more potent than suramin, which inhibited only 60% of angiogenesis at 125 nM. 10-Hydroxycamptothecin, administered orally at low doses of 2.5-7.5 mg/kg every two days, caused significant growth inhibition in Colo205 xenograft mice, but no acute toxicity. LD50: 104 mg/kg in mice (intraperitoneal injection).
IC 50
0.31 μm
References
[1] vladu b, woynarowski jm, manikumar g, wani mc, wall me, von hoff dd, wadkins rm. 7- and 10-substituted camptothecins: dependence of topoisomerase i-dna cleavable complex formation and stability on the 7- and 10-substituents. mol pharmacol. 2000 feb;57(2):243-51.
[2] xiao d, tan w, li m, ding j. antiangiogenic potential of 10-hydroxycamptothecin. life sci. 2001 aug 24;69(14):1619-28.
[3] ping yh, lee hc, lee jy, wu ph, ho lk, chi cw, lu mf, wang jj. anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. oncol rep. 2006 may;15(5):1273-9.
10-Hydroxycamptothecin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18747 | 58 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29885 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3772 | 58 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 17254 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2993 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
Related articles
- Camptothecin vs. 10-Hydroxycamptothecin: Uses and Synthesis
- Camptothecin (CPT) is a pentacyclic natural alkaloid extracted from Camptotheca acuminata. CPT is a unique class of complex qu....
- Nov 12,2024
View Lastest Price from 10-Hydroxycamptothecin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-14 | Hydroxycamptothecin
19685-09-7
|
US $0.00-0.00 / g | 1g | 98% | 2000 | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-11-13 | (S)-10-Hydroxycamptothecin
19685-09-7
|
US $44.00-84.00 / mg | 99.81% | 10g | TargetMol Chemicals Inc. | ||
 |
2023-02-24 | 10-Hydroxycamptothecin
19685-09-7
|
US $0.00 / mg | 5mg | ≥98%(HPLC) | 10 g | Shanghai Standard Technology Co., Ltd. |
-

- Hydroxycamptothecin
19685-09-7
- US $0.00-0.00 / g
- 98%
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- (S)-10-Hydroxycamptothecin
19685-09-7
- US $44.00-84.00 / mg
- 99.81%
- TargetMol Chemicals Inc.
-

- 10-Hydroxycamptothecin
19685-09-7
- US $0.00 / mg
- ≥98%(HPLC)
- Shanghai Standard Technology Co., Ltd.
19685-09-7(10-Hydroxycamptothecin)Related Search:
1of4