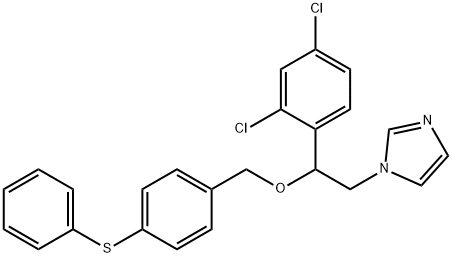
Fenticonazole
- CAS No.
- 72479-26-6
- Chemical Name:
- Fenticonazole
- Synonyms
- Fenticonazol;FENTICONAZOLE;Fenticonazole Nitrate-d9;1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio);1-[β-[[4-(Phenylthio)benzyl]oxy]-2,4-dichlorophenethyl]-1H-imidazole;1-[2,4-Dichloro-β-[[4-(phenylthio)benzyl]oxy]phenethyl]-1H-imidazole;1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-1H-imidazole;1H-Imidazole, 1-[2-(2,4-dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-;1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-1H-imidazole USP/EP/BP;fenticonazole:1-(2-(2,4-dichlorophenyl)-2-((4-(phenylthio)phenyl)methoxy)ethyl)-1h-IMIDAZOLE
- CBNumber:
- CB4668990
- Molecular Formula:
- C24H20Cl2N2OS
- Molecular Weight:
- 455.4
- MDL Number:
- MFCD00865611
- MOL File:
- 72479-26-6.mol
| CAS DataBase Reference | 72479-26-6(CAS DataBase Reference) |
|---|---|
| FDA UNII | QG05NRB077 |
| ATC code | D01AC12,G01AF12 |
Fenticonazole price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | F279252 | FenticonazoleNitrate-d9 | 72479-26-6 | 1mg | $195 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0007259 | FENTICONAZOLE 95.00% | 72479-26-6 | 100MG | $582.12 | 2021-12-16 | Buy |
| AK Scientific | K232 | Fenticonazole | 72479-26-6 | 5g | $643 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0007259 | FENTICONAZOLE 95.00% | 72479-26-6 | 250MG | $813.95 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0007259 | FENTICONAZOLE 95.00% | 72479-26-6 | 1G | $1137.15 | 2021-12-16 | Buy |
Fenticonazole Chemical Properties,Uses,Production
Originator
Lomexin,Effik
Uses
Isotope labelled Fenticonazole Nitrate broad spectrum antimycotic; also active as antibacterial. Antifungal (topical).
Uses
Antifungal.
Definition
ChEBI: 1-[2-(2,4-dichlorophenyl)-2-{[4-(phenylsulfanyl)benzyl]oxy}ethyl]imidazole is a member of the class of imidazoles that carries a 2-(2,4-dichlorophenyl)-2-{[4-(phenylsulfanyl)benzyl]oxy}ethyl group at position 1. It is a member of imidazoles, a dichlorobenzene, an ether and an aryl sulfide.
Indications
Topical treatment of mycoses of the skin induced or sustained by fungi such as dermatophytes and yeasts. In addition, fenticonazole is indicated for therapy of vulvovaginal mycoses caused by Candida species and Candida (Torulopsis) glabrata.
Manufacturing Process
1-(2',4'-Dichlorophenyl)-2-chloroethanol:
49.5 g of sodium borohydride were added slowly and in small parts to a
suspension of 233 g of 1-(1'-hydroxy-2'-chloroethyl)-2,4-dichlorobenzene in 1
liter of methanol stirred at room temperature. The solution thus obtained was
stirred at room temperature for a further two hours, and it was then poured
into 1 liter of 5 N hydrochloric acid cooled with ice. After extraction with ethyl
acetate or chloroform, the extract was washed with water, with 1 N sodium
hydroxide, then again with water until neutrality, and finally with a saturated
sodium chloride solution. The extract was dried, the solvent evaporated off
and 220 g of an oil were obtained. The oil solidified on standing and the solid
1-(2',4'-dichlorophenyl)-2-chloro-ethanol melted at 48-51°C.
1-(2',4'-Dichlorophenyl)-2-(N-imidazolyl)ethanol:
30 g of sodium were added to a solution of 88.5 g of imidazole in 600 ml of
methanol; the solvent was then evaporated off. The residue was dissolved in
300 ml of dimethylformamide and heated to 115-120°C. To the solution so
obtained was added, dropwise and under stirring, a solution of 225 g of 1-
(2',4'-dichlorophenyl)-2-chloroethanol in 400 ml of dimethylformamide. The
mixture was heated to 115-120°C and maintained at that temperature for 20
min and, after subsequent cooling to 40°C, 2500 ml of iced water were added
under vigorous stirring. The product precipitated under stirring over a period
of about two hours, the upper liquid was then decanted off, a further 2500 ml
of water were added and, after standing, the whole was filtered. The
precipitate thus obtained was dried and crystallized from toluene. 170 g of the
1-(2',4'-dichlorophenyl)-2-(N-imidazolyl)ethanol, melting at 134-135°C, was
obtained.
METHOD 1:
A solution of 2.57 g of 1-(2',4'-dichlorophenyl)-2-(N-imidazolyl)ethanol in 10
ml of hexamethylphosphoramide was dropped at 25°C into a suspension of
0.52 g of sodium hydride (50% in oil) in 5 ml of hexamethylphosphoramide.
When hydrogen emission was over, the salification was completed by heating
for 1 hour at 50°C. After cooling to 25°C, 2.58 g of 1-chloromethyl-4-
phenylthiobenzene were added. The temperature was raised to 50°C and
maintained at that temperature for 12 hours. At the end of the reaction, the
mixture was poured into 200 ml of water, the product was extracted with
diethyl ether, the solvent was evaporated off and the residue was purified
twice on a silica gel column, using ethyl acetate as eluant and testing the
various fractions by TLC. The solvent was evaporated off the middle fractions to give 2.4 g of the 1-[2,4-dichloro-beta-[[p-(phenylthio)benzyl]oxy]
phenethyl]imidazole as a yellowish oil, showing a single spot on TLC.
METHOD 2:
0.66 g of sodium hydride (50% in oil) were added at 20-30°C and under
nitrogen atmosphere to 3.86 g of 1-(2',4'-dichlorophenyl)-2-(N-imidazolyl)
ethanol in 15 ml of dimethylsulphoxide (dried on calcium hydride). The
mixture was heated under stirring at 50-60°C until gas emission was over.
After cooling to 20-25°C, 0.5 g of potassium iodide were added and slowly a
solution of 3.51 g of 1-chloromethyl-4-phenylthiobenzene in 4 ml of
dimethylsulphoxide was dropped in. The mixture was stirred at 20-25°C until
addition of the 1-chloromethyl-4-phenylthiobenzene was over. The mixture
was then poured into 150 ml of water and extracted with diethyl ether. To the
etheric solution, after drying on anhydrous sodium sulphate, was added
excess 4 N nitric acid solution in diethyl ether: the desired product
precipitated as nitrate, an oil which solidified on standing. After standing for
20 hours, the etheric liquid was decanted off and the residue was crystallized
from ethanol. The nitrate thus obtained, not completely pure, was dissolved in
water and excess sodium carbonate was added in order to liberate the base
which was then extracted with ethyl acetate. The base, obtained by filtration,
was purified on a silica gel column using ethyl acetate as eluant. The
combined fractions containing the desired product were evaporated to
dryness. The residue was dissolved in diethyl ether, again transformed into
the nitrate and crystallized from ethanol. Yield: 3.1 g of a white crystalline
powder, melting at 136°C; λmax 252 nm (methanol).
Therapeutic Function
Antifungal
Side effects
Local irritations such as itching and burning sensations and allergic reactions may occur in rare cases and are mainly due to the galenic formulation.
Solubility in organics
Solubility at 20 ?C: water < 0.1 mg/mL, diethyl ether < 0.1 mg/mL, ethanol 30 mg/mL, methanol 100 mg/mL, chloroform 300 mg/mL, DMF 600 mg/mL.
Fenticonazole Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22883 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| Suzhou ARTK Medchem Co., Ltd. | +86-512-68323658 +86-18168183658 | sales1@artkmedchem.com | China | 39004 | 58 |
| TargetMol Chemicals Inc. | +8613564774135 | zijue.cai@tsbiochem.com | United States | 19885 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32343 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23912 | 58 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2123 | 70 |
View Lastest Price from Fenticonazole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Fenticonazole
72479-26-6
|
US $1520.00-1980.00 / mg | 10g | TargetMol Chemicals Inc. | |||
![1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-1H-imidazole USP/EP/BP pictures](/ProductImageEN/2021-07/Small/96664c37-7907-4302-9d87-a7f170c6fb57.jpg) |
2021-07-20 | 1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-1H-imidazole USP/EP/BP
72479-26-6
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Fenticonazole
72479-26-6
- US $1520.00-1980.00 / mg
- TargetMol Chemicals Inc.
-
![1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-1H-imidazole USP/EP/BP pictures](/ProductImageEN/2021-07/Small/96664c37-7907-4302-9d87-a7f170c6fb57.jpg)
- 1-[2-(2,4-Dichlorophenyl)-2-[[4-(phenylthio)phenyl]methoxy]ethyl]-1H-imidazole USP/EP/BP
72479-26-6
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited