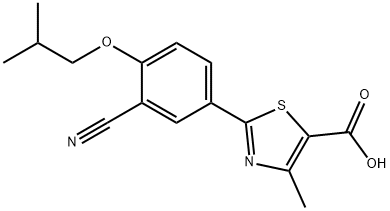
Febuxostat
- CAS No.
- 144060-53-7
- Chemical Name:
- Febuxostat
- Synonyms
- FBX;Uloric;Febuxostat Impurity;2-(3-cyano-4-isobutoxyphenyl);TMX 67;CS-1598;Tei-6720;NSC63871;Febrista;Adenuric
- CBNumber:
- CB4841564
- Molecular Formula:
- C16H16N2O3S
- Molecular Weight:
- 316.37
- MDL Number:
- MFCD00871598
- MOL File:
- 144060-53-7.mol
- MSDS File:
- SDS
| Melting point | 238-239°(dec.) |
|---|---|
| Boiling point | 536.6±60.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 2.48±0.10(Predicted) |
| color | White to Off-White |
| Merck | 14,3948 |
| InChI | InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20) |
| InChIKey | BQSJTQLCZDPROO-UHFFFAOYSA-N |
| SMILES | S1C(C(O)=O)=C(C)N=C1C1=CC=C(OCC(C)C)C(C#N)=C1 |
| CAS DataBase Reference | 144060-53-7(CAS DataBase Reference) |
| FDA UNII | 101V0R1N2E |
| NCI Drug Dictionary | febuxostat |
| ATC code | M04AA03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501 | |||||||||
| RTECS | XJ3675310 | |||||||||
| HS Code | 2934.10.2000 | |||||||||
| NFPA 704 |
|
Febuxostat price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1285 | Febuxostat 98.5-102.0% (HPLC) | 144060-53-7 | 1G | $202 | 2023-06-20 | Buy |
| TCI Chemical | F0847 | Febuxostat >97.0%(HPLC)(T) | 144060-53-7 | 1g | $98 | 2024-03-01 | Buy |
| TCI Chemical | F0847 | Febuxostat >97.0%(HPLC)(T) | 144060-53-7 | 5g | $316 | 2024-03-01 | Buy |
| Cayman Chemical | 14127 | Febuxostat ≥98% | 144060-53-7 | 5mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 14127 | Febuxostat ≥98% | 144060-53-7 | 10mg | $107 | 2024-03-01 | Buy |
Febuxostat Chemical Properties,Uses,Production
Description
Febuxostat, a selective xanthine oxidase inhibitor, was launched for the chronic management of hyperuricemia in patients with gout. Hyperuricemia is defined as a serum uric acid concentration exceeding the limit of solubility. It predisposes affected persons to gout, a disease characterized by the formation of crystals of monosodium urate or uric acid from supersaturated fluids in joints and other tissues. Crystal deposition is asymptomatic, but it is revealed by bouts of joint inflammation. If left untreated, further crystals accumulate in joints and can form deposits known as tophi. A major aim in gout management is the long-term reduction of serum uric acid concentrations below saturation levels, as this results in crystal dissolution and eventual disappearance.
Febuxostat is a nonpurine derivative with higher potency and selectivity than allopurinol for inhibiting xanthine oxidase. It completely inhibits human xanthine oxidase activity in the lung cancer cell line A549, whereas the activities of other enzymes involved in purine or pyrimidine metabolism (e.g., purine nucleoside phosphorylase, adenosine deaminase, and pyrimidine nucleoside phosphorylase) are affected by<4%.
Chemical Properties
Crystalline Solid
Physical properties
Febuxostat has low solubility. It is almost insoluble in acidic conditions, slightly soluble in neutral conditions, and slightly more soluble in alkaline conditions. It is not suitable for making injections, but it can be taken orally because of its high oil-water partition coefficient and strong ability to cross cell membranes.
Originator
Teijin (Japan)
Uses
Febuxostat is a new generation xanthine oxidase inhibitor developed by Tejin Co. (Japan,) used clinically for for long-term treatment of hyperuicemia (gout,) a new and highly effective non-purine selective inhibitor of xanthine oxidase. 40-120 mg/day febuxostat was proven effective in lowering serum urate levels when administered to manage hyperuricemia in patients with gout. It is not recommended for gout patients without hyperuricemia.
Preparation
Febuxostat can be synthesized in a multistep sequence from 2,4-dicyanophenol, starting with the alkylation of the phenolic hydroxyl group with isobutyl bromide and potassium carbonate, followed by treatment with thioacetamide in hot dimethyl formamide to yield 3-cyano-4-isobutoxythiobenzamide. Cyclization of the thioamide group with 2-chloroacetoacetic acid ethyl ester in refluxing ethanol affords 2-(3-cyano-4-isoutoxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester, which is hydrolyzed with sodium hydroxide to produce febuxostat.
Definition
ChEBI: Febuxostat is a 1,3-thiazolemonocarboxylic acid that is 4-methyl-1,3-thiazole-5-carboxylic acid which is substituted by a 3-cyano-4-(2-methylpropoxy)phenyl group at position 2. It is an orally-active, potent, and selective xanthine oxidase inhibitor used for the treatment of chronic hyperuricaemia in patients with gout. It has a role as an EC 1.17.3.2 (xanthine oxidase) inhibitor. It is an aromatic ether, a nitrile and a 1,3-thiazolemonocarboxylic acid.
brand name
Uloric, Adenuric
General Description
Febuxostat is a potent, non-purine compound, which inhibits the expression of cytokines/chemokines. It has also been reported to inhibit LPS-induced TNF-α, VCAM-1, MMP9 and MCP-1 expression.
Biological Activity
Febuxostat is an antihyperuricemic nonpurine inhibitor of both the oxidized and reduced forms of xanthine oxidase. It inhibits bovine milk xanthine oxidase as well as mouse and rat liver xanthine oxidase/xanthine dehydrogenase (IC50s = 1.4, 1.8, and 2.2 nM, respectively). It is 10-30 times more potent than the hypoxanthine analog allopurinol (; Kis = 0.7 nM and 0.7 μM, respectively). Febuxostat decreases the serum level of urate in a potassium oxonate rat model of hyperuricemia (ED50 = 1.5 mg/kg). It reduces hepatic macrovesicular steatosis in mice fed a high-fat diet containing trans fatty acids when administered at a dose of 1 mg/kg per day. Febuxostat (0.75 mg/kg) also increases CNS expression of glutamate oxaloacetate transaminase 2 (GOT2) and improves neurological symptoms in a mouse model of secondary progressive experimental autoimmune encephalomyelitis (EAE). Formulations containing febuxostat have been used in the treatment of symptomatic hyperuricemia in patients with gout.
Biochem/physiol Actions
Febuxostat is a potent non-purine xanithine oxidase inhibitor. Febuxostat is used in urate lowering therapies (ULTs) for the treatment of gout.
Clinical Use
Fabuxostat was discovered by Teijin Pharmaceuticals and licensed to TAP Pharmaceuticals (which is currently part of Takeda Pharmaceuticals) and was approved in the U.S. for the treatment of hyperuricemia in patients with gout. It is a once-daily non-purine based agent with potent inhibitory activity against xanthine oxidase. The safety profile of the drug also does not require dose adjustment for patients with mild to moderate renal or hepatic impairment. Febuxostat is the first new agent cleared for this indication in 40 years.
Side effects
The incidence of adverse events such as dizziness, diarrhea, headache, and nausea with febuxostat was similar to allopurinol. Febuxostat is contraindicated in patients being treated with the xanthine oxidase substrates such as azathioprine, mercaptopurine, and theophylline.
Synthesis
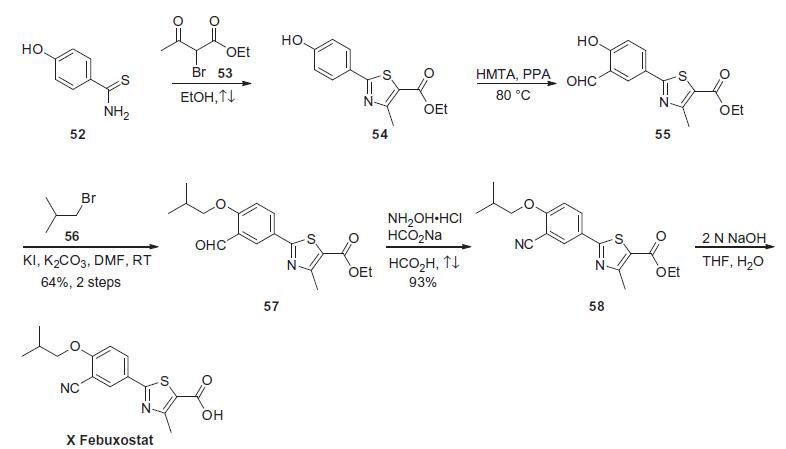
There are a number of routes available to prepare this agent as discussed in recent publications. The synthesis shown in Scheme 10 is a short and concise route and does not require the use of toxic reagents. Thus the commercially available and easily prepared 4-hydroxythiobenzamide (52) was reacted with ethyl bromoacetoacetate (53) in refluxing ethanol to provide the thiazole ester 54 in ??60% yield after crystallization. The phenolic ester 54 was then treated with hexamethylenetetramine (HMTA) in polyphosphoric acid at 80 ??C to provide the crude aldehyde 55 (74% conversion by HPLC). Reaction of phenol 55 and isobutyl bromide (56) in the presence of potassium carbonate with catalytic potassium iodide in DMF gave isobutyl ether 57 (64%, two steps). This ether was then converted in one pot to nitrile 58 in 93% by reacting the aldehyde with hydroxylamine hydrochloride and sodium formate in refluxing formic acid. Saponification of the ester 58 with aqueous sodium hydroxide provided fabuxostat (X).
Drug interactions
Potentially hazardous interactions with other drugs Azathioprine: avoid concomitant use, increased risk of neutropenia. Cytotoxics: avoid concomitant use with mercaptopurine. Theophylline: use with caution
Metabolism
Extensively metabolised by conjugation via the uridine diphosphate glucuronosyltransferase (UDPGT) enzyme system, and by oxidation via the cytochrome P450 isoenzyme system to form active metabolites. About 49% of a dose is excreted via the urine, and 45% via the faeces (12% as unchanged drug)
storage
Store at RT
Mode of action
Xanthine oxidase is the main enzyme promoting uric acid production. It works by non-competitively blocking the molybdenum pterin center, which is the active site of xanthine oxidase. Through highly selective inhibition of oxidized and reduced xanthine oxidase, Febuxostat can reduce the synthesis of uric acid, decreasing its concentration and effectively treating gout. Through liver metabolism, Xanthine oxidase does not rely on renal excretion, so patients with moderate to severe liver and kidney dysfunction do not need to reduce dosages. Febuxostat is a non-purine XOR inhibitor, so it is very safe.
Febuxostat Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SHANDONG BOYUAN PHARMACEUTICAL CO., LTD. | +86-0531-69954981 +8615666777973 | dwyane.wang@boyuanpharm.com | China | 211 | 58 |
| shan dong Fengjin Pharmaceutical company | +8615066764791 | liangfulin@fengjin-pharma.com | China | 8 | 58 |
| JUYE XIANDAI fine chemistry Co.,Ltd | +86-18958038633; +8618958038633 | tony@sdhypharma.com | China | 64 | 58 |
| Hubei Chuyunshun Biotechnology Co., Ltd. | +86-15926415536 +86-15926415536 | hbcyssw@163.com | China | 160 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12834 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 970 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 456 | 58 |
Related articles
- Febuxostat: Application, Mechanism of Action and Side Effects
- Febuxostat is a medication used to manage and treat hyperuricaemia and gout. It is also a xanthine oxidase inhibitor.
- Mar 26,2024
Related Qustion
- Q:Why is febuxostat better than allopurinol?
- A:The cardiovascular safety of febuxostat compared to allopurinol for treating gout remains equivocal. Febuxostat had a better s....
- Nov 11,2024
View Lastest Price from Febuxostat manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-23 | Febuxostat
144060-53-7
|
US $150.00 / kg | 1kg | 99% | 500kg | Hebei Zhuanglai Chemical Trading Co Ltd | |
 |
2024-12-20 | Febuxostat
144060-53-7
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-12-20 | Febuxostat
144060-53-7
|
US $0.00 / kg | 1kg | 99%min | 20ton | Jinan Jianfeng Chemical Co., Ltd |
-

- Febuxostat
144060-53-7
- US $150.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co Ltd
-

- Febuxostat
144060-53-7
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Febuxostat
144060-53-7
- US $0.00 / kg
- 99%min
- Jinan Jianfeng Chemical Co., Ltd
144060-53-7(Febuxostat)Related Search:
1of4