Perchloryl fluoride
- CAS No.
- 7616-94-6
- Chemical Name:
- Perchloryl fluoride
- Synonyms
- Chloryl fluoroxide;Perchloryl fluoride;Chlorine oxyfluoride;Chlorine trioxide fluoride;Hyperchloric acid fluoride;Fluorochlorine(VII) trioxide;Perchloryl fluoride ((ClO3)F)
- CBNumber:
- CB4852216
- Molecular Formula:
- ClFO3
Lewis structure
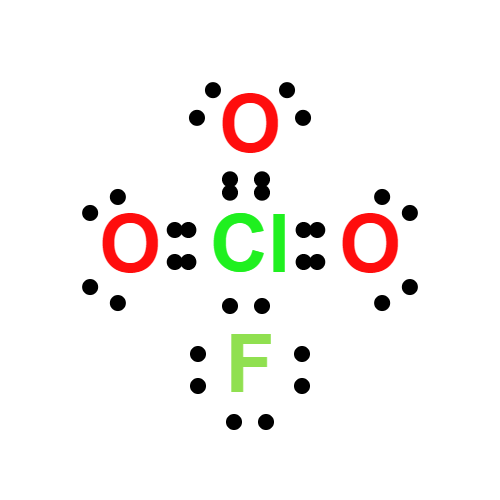
- Molecular Weight:
- 102.45
- MDL Number:
- MOL File:
- 7616-94-6.mol
| Melting point | -147.7° |
|---|---|
| Boiling point | bp -46.7° |
| Density | d20 (liq) 1.434 |
| vapor pressure | 1192.419kPa at 25℃ |
| form | colorless gas |
| color | Colorless, noncorrosive gas |
| Odor | characteristic sweet odor |
| EWG's Food Scores | 1 |
| FDA UNII | 73NC8XM516 |
| EPA Substance Registry System | Perchloryl fluoride (7616-94-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H314-H270-H330 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P220-P244-P370+P376-P403-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501 |
| RIDADR | 3157 |
| OEB | A |
| OEL | TWA: 3 ppm (14 mg/m3), STEL: 6 ppm (28 mg/m3) |
| HazardClass | 2.2 |
| Toxicity | LCLo ihl-rat: 2000 ppm/40M TXAPA9 27,527,74 |
| IDLA | 100 ppm |
Perchloryl fluoride Chemical Properties,Uses,Production
Chemical Properties
The perchloryl fluoride,FClO3, the acyl fluoride of perchloric acid, is a stable compound. It can be prepared by electrolysis of a saturated solution of sodium perchlorate in anhydrous hydrofluoric acid.
Chemical Properties
Perchloryl fluoride is a colorless gas. Characteristic sweet odor. Shipped as a liquefied compressed gas.
Uses
perchloryl fluoride's uses are as an effective fluorinating agent, as an oxidant in rocket fuels, and as a gaseous dielectric for transformers.
Uses
In organic synthesis to introduce F atoms into organic molecules; oxidizing agent in rocket fuels; insulator for high-voltage systems
Uses
In organic synthesis to introduce fluorine atoms into organic molecules. As oxidizing agent; insulator for high voltage systems.
General Description
Perchloryl fluoride is a colorless, non corrosive gas with a characteristic sweet odor. Contact with the material may cause irritation to skin, eyes, and mucous membranes. Perchloryl fluoride is very toxic by inhalation and skin absorption. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Perchloryl fluoride is a propellant; a powerful oxidant. Perchloryl fluoride ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174].
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution.
Fire Hazard
Substance does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
A poison gas which forms methemoglobin in the body and destroys red cells causing anemia, anorexia, and cyanosis. Recovery is said to be rapid, leaving no permanent physiological damage. Can be absorbed through the skin. Its odor can be detected as low as 10 ppm although this cannot be relied upon as an indication of toxic concentration in air. While nonflammable, it supports combustion. It is a powerful oxidzer. Moderately explosive. Potentially explosive reactions with combustible gases or vapors. benzene + aluminum trichloride, benzocyclobutene + butyllithium + potassium tert-butoxide, calcium acetylide, potassium cyanide, potassium thiocyanate, sodium iochde, charcoal, ethyl-4-fluorobenzoylacetate, hydrocarbons, hydrogen sulfide, nitrogen oxide, sulfur dichloride, vinylidene chloride, 3a-hydroxy-5p-androstane-1 1,17-di0ne-l 7hydrazone, lithiated compounds, 2lithio (dimet hy laminomethyl)f erroxene, methyl-2-bromo-5,5-ethylene dioxy(2,2,1)bicycloheptane-7-carboxylate, aliphatic heterocyclic amines, sodium methoxide + methanol, vinylidene chloride. Reacts to form explosive products with nitrogenous bases (e.g., isopropylamine, isobutylamine, aniline, phenyl hydrazine, 1,2-diphenyl hydrazine), sawdust, lampblack. Violent reaction with finely dwided organic materials. A fluorinating agent in chemical synthesis, and an oxidant in rocket fuel. When heated to decomposition it emits toxic fumes of Fand Cl-. See also FLUORINE and PERCHLORATES
Potential Exposure
Perchloryl fluoride has been used as a liquid oxidant in rocket propellant combinations; as an insulating gas in high voltage electrical systems; as a fluorinating agent in organic synthesis.
Shipping
UN3083 Perchloryl fluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, Inhalation Hazard Zone B. UN3157 Liquefied gas, oxidizing, n.o.s., Hazard Class: 2.2; Labels: 2.2-Non-flammable compressed gas, 5.1-Oxidizer, Technical Name Required. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
A strong oxidizing gas. Violent reaction with benzene, calcium hydride; combustibles, olefins, strong bases; sulfur, sulfuric acid; amines, reducing agents; alcohols. Contact with carbonaceous materials (such as charcoal) or finely divided metals (such as powdered magnesium and aluminum, zinc) are a fire and explosion hazard. Attacks some plastics, rubber, and coatings
Waste Disposal
Return refillable compressed gas cylinders to supplier. Incineration together with flammable solvent in furnace equipped with afterburner and scrubber.
Perchloryl fluoride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Golden Pharma Co., Limited | +undefined18958062155 | sales@zjgoldpharm.com | China | 5905 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Supplier | Advantage |
|---|---|
| Hubei xin bonus chemical co. LTD | 58 |
| Golden Pharma Co., Limited | 58 |
| Mainchem Co., Ltd. | 55 |





