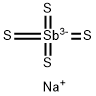SODIUM THIOANTIMONATE
- CAS No.
- 13776-84-6
- Chemical Name:
- SODIUM THIOANTIMONATE
- Synonyms
- SCHLIPPE'S SALT;sodium thioantimonide;SODIUM SULFANTIMONATE;SODIUM THIOANTIMONATE;Sodium sulfoantimonate;Sodium Thioantimonate(V);Trinatriumtetrathioantimonat;Sodiumthioantimonat-9-hydrate;trisodium tetrathioantimonate;trisodium,sulfanylideneantimony,trihydroxide
- CBNumber:
- CB5125131
- Molecular Formula:
- NaS4Sb-2
- Molecular Weight:
- 272.99
- MDL Number:
- MFCD00046203
- MOL File:
- 13776-84-6.mol
| Melting point | 87℃ [CRC10] |
|---|---|
| Boiling point | decomposes 234℃ [CRC10] |
| Density | 1.806 |
| Water Solubility | g anhydrous/100 g H2O: 13.4 (0°C), 27.9 (20°C), 88.3 (80°C) [LAN05]; insoluble alcohol; decomposed by weak acids [MER06] |
| FDA UNII | W0I6VDX5GA |
| EPA Substance Registry System | Antimonate(3-), tetrathioxo-, trisodium, (T-4)- (13776-84-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H332 |
| Precautionary statements | P261-P271-P304+P340-P312-P264-P270-P301+P312-P330-P501 |
SODIUM THIOANTIMONATE Chemical Properties,Uses,Production
Chemical Properties
colorless or light yellow large crystal(s); becomes covered with reddish brown coating of antimony sulfide if exposed to air [MER06]
Synthesis
A solution of 15 g. of SbCl3
in 600 ml. of dilute hydrochloric
acid is prepared. If a precipitate is produced as a consequence
of hydrolysis, concentrated hydrochloric acid is added until the
solution becomes clear. Then H2S is bubbled through the solution,
and the precipitate of Sb2S3 is filtered off. It is mixed with 60 ml.
of 20% NaOH; 6 g. of S (powder form) is added and the mixture is
boiled with constant stirring until the orange-red color turns
yellow. The water lost on boiling should be replaced from time
to time. The solution is filtered through a fluted filter and evaporated until crystallization begins. If the solution becomes
turbid, a few drops of 20% NaOH are added until it clears. After
complete cooling, the crystalline precipitate is suction-filtered,
washed with some alcohol, and dried in a desiccator over quicklime
to which a few drops of ammonium sulf ide solution have been added.
The mother liquor can be further concentrated. The preparation
can be purified by recrystallization from weakly alkaline solution
(a few milliliters of sodium hydroxide are added to the water).
Sb2S3 + 8 NaOH + 6 S = 2 Na3SbS4 + Na2SO4 + 4 H2O
Purification Methods
Crystallise it from warm water (2mL/g made weakly alkaline with a few drops of dilute aqueous NaOH) by cooling to 0o. It forms a yellow nonahydrate which readily effloresces in air. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 619 1963.]
SODIUM THIOANTIMONATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8672 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Shaanxi Didu New Materials Co. Ltd | 58 |