FLUTICASONE FUROATE
- CAS No.
- 397864-44-7
- Chemical Name:
- FLUTICASONE FUROATE
- Synonyms
- AvaMys;Veramyst;AllerMist;Gw 685698x;GSK 685 698;Unii-js86977wnv;FLUTICASONE FUROATE;Fluticasone Foruate;Fluticasone Furoate-d3;Fluticasone Impurity 31 (Fluticasone Furoate)
- CBNumber:
- CB51509114
- Molecular Formula:
- C27H29F3O6S
- Molecular Weight:
- 538.58
- MDL Number:
- MFCD09954122
- MOL File:
- 397864-44-7.mol
- MSDS File:
- SDS
| Melting point | 250-252°C (dec.) |
|---|---|
| Boiling point | 625.2±55.0 °C(Predicted) |
| Density | 1.39 |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly, Heated, Sonicated), Methanol (Slightly) |
| pka | 12.52±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| FDA UNII | JS86977WNV |
| ATC code | R01AD12,R03BA09 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361d |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
FLUTICASONE FUROATE price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML3103 | Fluticasone furoate ≥98% (HPLC) | 397864-44-7 | 10MG | $89 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML3103 | Fluticasone furoate ≥98% (HPLC) | 397864-44-7 | 50MG | $359 | 2024-03-01 | Buy |
| Cayman Chemical | 29878 | Fluticasone Furoate | 397864-44-7 | 5mg | $116 | 2024-03-01 | Buy |
| Cayman Chemical | 29878 | Fluticasone Furoate | 397864-44-7 | 25mg | $343 | 2024-03-01 | Buy |
| Cayman Chemical | 29878 | Fluticasone Furoate | 397864-44-7 | 1mg | $32 | 2024-03-01 | Buy |
FLUTICASONE FUROATE Chemical Properties,Uses,Production
Description
Fluticasone furoate is a new corticosteroid derivative launched as a
nasal spray for the treatment of seasonal and perennial allergic rhinitis in adults
and in children aged ≥2 years. It is structurally closely related to the previously
marketed intranasal corticosteroid fluticasone propionate (FP). Both of these
steroids contain the unusual S-fluoromethyl carbothioate group, which confers
high lipophilicity and hence enhanced binding and retention of the drug by the
nasal tissue. Additionally, the carbothioate group rapidly undergoes first-pass
metabolism by CYP3A4, thus minimizing systemic exposure of the parent drug.
Fluticasone furoate is a potent ligand for the glucocorticoid receptor (GR), with
a relative receptor affinity (RRA) of 2,989 with reference to dexamethasone RRA
of 100.
Following intranasal administration, most of the dose is eventually swallowed and undergoes incomplete absorption and extensive firstpass metabolism in the liver and gut, resulting in negligible systemic exposure. Pharmacokinetic studies using oral solution dosing and intravenous dosing show that at least 30% of fluticasone furoate is absorbed and then rapidly cleared from plasma. The most common adverse reactions (W1% incidence) included headache, epistatix, sinus and throat pain, nasal ulceration, back pain, pyrexia, and cough. Fluticasone furoate is chemically derived starting from a readily available corticosteroid, 6a,9a-difluoro-11b,17a-dihydroxy-16a-methyl-3-oxoandrosta-1,4-diene-17b-carboxylic acid, by first converting the carboxylic acid group to the corresponding carbothioic acid via activation with carbonyl diimidazole followed by reaction with hydrogen sulfide gas. Subsequently, selective acylation of the 17a-hydroxyl group with 2-furoyl chloride and alkylation of the 17b-carbothioic acid group with bromofluoromethane under basic conditions provides fluticasone furoate.
Description
Fluticasone furoate is a synthetic glucocorticoid. It is selective for the glucocorticoid receptor over the mineralocorticoid, progesterone, and androgen receptors in reporter assays (EC50s = 0.03, 23.4, 0.9, and >10,000 nM, respectively), as well as estrogen receptor α (ERα) and ERβ in scintillation proximity assays (EC50s = >10,000 nM for both). Fluticasone furoate reduces LPS-induced increases in TNF-α production in human peripheral blood mononuclear cells (PBMCs) with an EC50 value of 0.12 nM. It decreases S. aureus enterotoxin-induced increases in IFN-γ, IL-2, IL-5, IL-17, and TNF-α levels in patient-derived nasal polyp tissue when used at a concentration of 100 nM. Intrathecal administration of fluticasone furoate (30 μg/animal) reduces ovalbumin-induced increases in bronchoalveolar lavage fluid (BALF) eosinophil infiltration in a rat model of allergic inflammation. Formulations containing fluticasone furoate have been used in the treatment of seasonal allergies.
Chemical Properties
White to Off-White Solid
Originator
GSK (US)
Uses
Fluticasone-d3 Furoate is an isotope labelled form of Fluticasone Furoate (F599510). Fluticasone Furoate is a synthetic corticosteroid derived from fluticasone for treatment of seasonal allergic rhinitis.
Uses
It is one of the newest
Definition
ChEBI: A trifluorinated corticosteroid that consists of 6alpha,9-difluoro-11beta,17alpha-dihydroxy-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-16-methyl-3-oxoandrosta-1,4-diene bearing a 2-furoyl s bstituent at position 17. Used in combination with vilanterol trifenate for treatment of bronchospasm associated with chronic obstructive pulmonary disease.
brand name
Veramyst
Synthesis
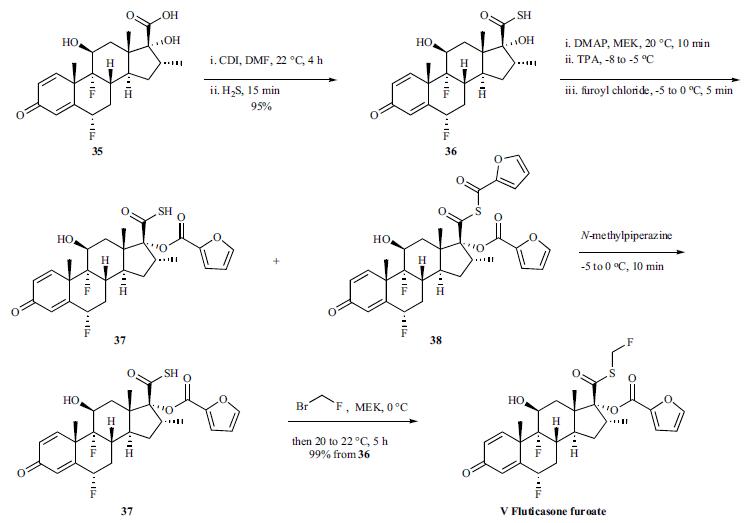
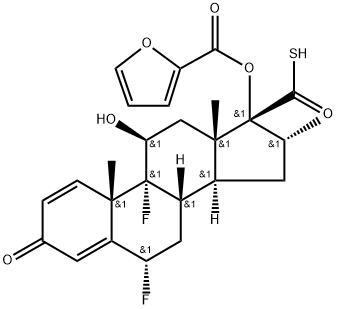
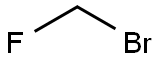
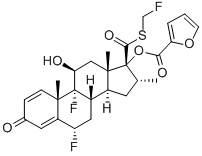
The synthesis of fluticasone on large scale was disclosed in the patent literature. The starting 6|á ,9|á- difluoro-11|?-17|á-dihydroxy-16|á-methyl-3-oxoandrosta-1,4- diene-17|?-carboxylic acid 35 was converted to the analogous carbothioic acid 36 in 95% yield via activation with carbonyl diimidazole, followed by reaction with hydrogen sulfide gas. Conversion of the carbothioic acid to fluticasone was completed through a three-step sequence in a one pot process in 99% overall yield. Carbothioic acid 36 and DMAP were dissolved in MEK. Tripropylamine (TPA) was then added to the mixture at -8 to -5??C. Neat furoyl chloride was then added dropwise over 2-3 minutes and the resulting mixture was then stirred at -5 to 0??C for 15 minutes generating a mixture of desired ester 37 and thioanhydride 38. A solution of N-methylpiperazine in water was then added dropwise over 2-3 minutes at -5 to 0??C to the crude reaction mixture and stirred for 10 minutes, which enabled the conversion of thioanhydride 38 to the ester 37. A solution of bromofluoromethane in MEK was then added rapidly at 0??C and the resulting solution was stirred at 20??C for 5 hours. After a simple work-up, fluticasone furoate (V) was obtained in 99% overall yield from 36 with 97% purity.
FLUTICASONE FUROATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21638 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| Lianyungang happen teng technology co., LTD | 15950718863 | wang666xt@163.com | CHINA | 295 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 | sales@czwytech.com | CHINA | 904 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Hebei shuoxi biotechnology co. LTD | +8613081092107 | CHINA | 964 | 58 | |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29815 | 58 |
View Lastest Price from FLUTICASONE FUROATE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-04 | Fluticasone furoate
397864-44-7
|
US $30.00-84.00 / mg | 99.58% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-10-24 | Fluticasone Furoate-d3 | US $0.00-0.00 / mg | 10g | TargetMol Chemicals Inc. | |||
 |
2024-08-30 | Fluticasone furoate
397864-44-7
|
US $65.00 / g | 10g/Bag | 98% | 100KG | Baoji Guokang Bio-Technology Co., Ltd. |
-

- Fluticasone furoate
397864-44-7
- US $30.00-84.00 / mg
- 99.58%
- TargetMol Chemicals Inc.
-

- Fluticasone Furoate-d3
- US $0.00-0.00 / mg
- TargetMol Chemicals Inc.
-

- Fluticasone furoate
397864-44-7
- US $65.00 / g
- 98%
- Baoji Guokang Bio-Technology Co., Ltd.





