Iopamidol
- CAS No.
- 60166-93-0
- Chemical Name:
- Iopamidol
- Synonyms
- isovue;niopam;b-15000;lamide);sq13396;iopamiro;Jopamiro;Solutras;iopamido;IPAMIDOL
- CBNumber:
- CB5384796
- Molecular Formula:
- C17H22I3N3O8
- Molecular Weight:
- 777.09
- MDL Number:
- MFCD00867931
- MOL File:
- 60166-93-0.mol
| Melting point | >3200C (dec) |
|---|---|
| alpha | D20 -2.01° (c = 10 in water) |
| Boiling point | 740.14°C (rough estimate) |
| Density | 2.0203 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Freely soluble in water, very slightly soluble in methanol, practically insoluble in ethanol (96 per cent) and in methylene chloride |
| form | Solid |
| pka | pKa (25°) 10.70 |
| color | White to off-white |
| Water Solubility | 473.7g/L(25 ºC) |
| CAS DataBase Reference | 60166-93-0(CAS DataBase Reference) |
| FDA UNII | JR13W81H44 |
| NCI Drug Dictionary | Isovue |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| HS Code | 2924296000 |
| Toxicity | LD50 in mice, rats, rabbits, dogs (g/kg): 44.5, 28.2, 19.6, 34.7 i.v. (Felder); LD50 in mice (mg iodine/kg body wt): 21,800 i.v.; 20,000 i.p.; 1500 intracerebral (Felder, Pitre). |
Iopamidol price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | I0329000 | Iopamidol European Pharmacopoeia (EP) Reference Standard | 60166-93-0 | i0329000 | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1344702 | Iopamidol | 60166-93-0 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR1985 | Iopamidol Pharmaceutical Secondary Standard; Certified Reference Material | 60166-93-0 | 500mg | $218 | 2024-03-01 | Buy |
| TRC | I735600 | Iopamidol | 60166-93-0 | 2mg | $60 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0003016 | IOPAMIDOL 95.00% | 60166-93-0 | 1MG | $152.25 | 2021-12-16 | Buy |
Iopamidol Chemical Properties,Uses,Production
Chemical Properties
White Crystalline Powder
Originator
Iopamiro,Bracco,Italy,1981
Uses
Iopamidol is an organic iodine compound and used as a nonionic radiocontrast medium. Diagnostic aid (radiopaque medium). Iopamidol blocks x-rays as they pass through the body, thereby allowing body structures not containing iodine to be visualized. The degree of opacity produced by iopamidol is directly proportional to the total amount of the iodinated contrast agent in the path of the x-rays. The visualization of body structures is dependent upon the distribution and elimination of iopamidol. (NCI05)
Definition
ChEBI: Iopamidol is a benzenedicarboxamide compound having N-substituted carbamoyl groups at the 1- and 3-positions, iodo substituents at the 2-, 4- and 6-positions and a (2S)-2-hydroxypropanamido group at the 5-position. It has a role as a radioopaque medium, an environmental contaminant and a xenobiotic. It is a benzenedicarboxamide, an organoiodine compound and a pentol.
Manufacturing Process
400 g (0.72 mol) 5-amino-2,4,6-triiodo-isophthalic acid was added to 200 ml
thionyl chloride, the mixture was stirred at a boil for 6 hours, and the
resulting solution was evaporated. The residue was dissolved in anhydrous
ethyl acetate, and the solution was again evaporated to dryness. The solid
material was dissolved in 4,000 ml ethyl acetate, and the solution was stirred
into an ice-cold solution of 500 g sodium chloride and 200 g sodium
bicarbonate in 2.5 liters water. The organic phase was separated from the
aqueous solution, washed with aqueous sodium solution, dried by contact with
anhydrous calcium chloride, and evaporated to dryness.
The residue of 420 g 5-amino-2,4,6-triiodo-isophthalyl chloride (97.5% yield)
had a melting point above 300°C when recrystallized from toluene.
300 g (0.503 mol) 5-amino-2,4,6-triiodo-isophthalyl chloride was dissolved in
1,200 ml dimethylacetamide, and 187 g (126 mol) DL-2-acetoxypropionyl
chloride was added dropwise to the solution with agitation. The mixture was
permitted to stand overnight at ambient temperature and was then
evaporated in a vacuum to approximately 400 ml. The oily residue was stirred
into ice water to precipitate 353 g crystalline DL-5-(α-acetoxypropionylamino)-
2,4,6-triiodo-isophthalyl chloride (98% yield) which was purified by
suspension in warm chloroform free alcohol.
The purified intermediate melted at 210°C. 70.9 g (0.10 mol) of the
intermediate was dissolved in 150 ml dimethylacetamide, and 15 g (0.08 mol)
tributylamine was added. The mixture was heated to 50°C, and 56.6 g (0.62
mol) 1,3-dihydroxyisopropylamine (2-amino-1,3-propanediol) dissolved in 80
ml dimethylacetamide was added drop by drop. The reaction went to
completion within a few hours, and the reaction mixture was evaporated to
dryness in a vacuum. The oily residue was added to 350 ml methylene
chloride with vigorous agitation, and the resulting precipitate was filtered off
and purified by repeated suspension of warm methylene chloride.
Work-up of the reaction mixture yielded 56.5 g (73.5%) DL-5-α-
hydroxypropionylamino-2,4,6-triiodo-isophthalic acid di-(1,3-
dihydroxyisopropylamide) which was recrystallized from aqueous ethanol and
melted with decomposition above 300°C.
brand name
Isovue (Bracco).
Therapeutic Function
Diagnostic aid (radiopaque medium)
General Description
Iopamidol is a low-osmolar, nonionicmonomer with 49% organically bound iodine. It is indicatedfor use in angiography, excretory urography, andnumerous CT procedures.
Side effects
Arm, back, or jaw pain; blurred vision; chest pain or discomfort; chest tightness or heaviness; confusion; dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position; fast or irregular heartbeat; feeling of warmth; hives; lightheadedness, dizziness, or fainting; nausea; redness of the face, neck, arms, and occasionally, upper chest; slow or irregular heartbeat; sudden sweating; trouble breathing; unusual tiredness or weakness
Toxicology
Serious skin reactions , including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis can occur with this medicine. Results from in vitro HEK293T cell-based assays indicate that iopamidol affects mitochondrial function Treatment with iopamidol induces ATP depletion, reduces the mitochondrial membrane potential, and elevates mitochondrial superoxide and reactive oxygen species accumulation.
Mode of action
Iopamidol is a Radiographic Contrast Agent. The mechanism of action of iopamidol is as a X-Ray Contrast Activity.

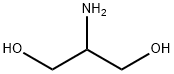
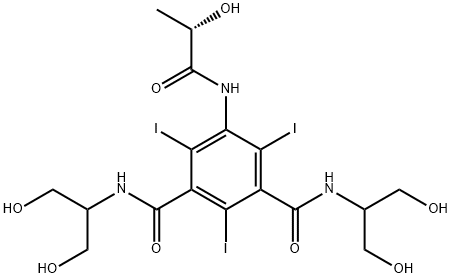
Iopamidol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xilinglab Co., Ltd. | +8618381337976 | bd@xilinglab.com | China | 86 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 13461 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23541 | 58 |
Related articles
- Iopamidol: properties, applications and safety
- Iopamidol is a widely used, safe contrast agent for medical imaging procedures, offering excellent visibility and patient comf....
- Nov 28,2023
- Iopamidol: Application in Imaging Diagnosis
- Iopamidol is clinically used in the angiography like cerebral arteriography. Cardioangiography includes coronary arteries, th....
- Oct 23,2019
View Lastest Price from Iopamidol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Iopamidol
60166-93-0
|
US $35.00-64.00 / mg | 99.47% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-08-14 | Iopamidol USP/EP/BP
60166-93-0
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2019-07-11 | Iopamidol
60166-93-0
|
US $1.00 / KG | 1KG | 98% | 1kg,5kg,100kg | Career Henan Chemical Co |
-

- Iopamidol
60166-93-0
- US $35.00-64.00 / mg
- 99.47%
- TargetMol Chemicals Inc.
-

- Iopamidol USP/EP/BP
60166-93-0
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Iopamidol
60166-93-0
- US $1.00 / KG
- 98%
- Career Henan Chemical Co
60166-93-0(Iopamidol)Related Search:
1of4





