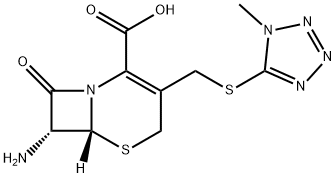
Cemandil sodium salt
- CAS No.
- 42540-40-9
- Chemical Name:
- Cemandil sodium salt
- Synonyms
- CEFAMANDOLE NAFATE;Fado;CefaM;Cedol;CeMado;Nafate;mandol;Cefiran;Kefadol;Pavecef
- CBNumber:
- CB5430833
- Molecular Formula:
- C19H17N6NaO6S2
- Molecular Weight:
- 512.49
- MDL Number:
- MFCD00864877
- MOL File:
- 42540-40-9.mol
| Melting point | 190-193°C |
|---|---|
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Freely soluble in water, sparingly soluble in methanol. |
| form | Solid |
| pka | 2.6-3.0(at 25℃) |
| color | White to Off-White |
| Water Solubility | Freely soluble in water. Sparingly soluble in methanol |
| CAS DataBase Reference | 42540-40-9(CAS DataBase Reference) |
| FDA UNII | 8HDO7941DO |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H317-H319-H334-H335 | |||||||||
| Precautionary statements | P261-P280-P284-P304+P340-P305+P351+P338-P342+P311 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 36/37/38-42/43 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XI0380000 | |||||||||
| HS Code | 2941906000 | |||||||||
| NFPA 704 |
|
Cemandil sodium salt price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C0682300 | Cefamandole nafate European Pharmacopoeia (EP) Reference Standard | 42540-40-9 | c0682300 | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1097400 | Cefamandole nafate United States Pharmacopeia (USP) Reference Standard | 42540-40-9 | 250mg | $412 | 2024-03-01 | Buy |
| Alfa Aesar | J66735 | Cefamandole nafate | 42540-40-9 | 5g | $168.65 | 2024-03-01 | Buy |
| Alfa Aesar | J66735 | Cefamandole nafate | 42540-40-9 | 10g | $336 | 2024-03-01 | Buy |
| Cayman Chemical | 23988 | Cefamandole Nafate ≥95% | 42540-40-9 | 1g | $88 | 2024-03-01 | Buy |
Cemandil sodium salt Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Mandokef,Lilly,W. Germany,1977
Uses
A second generation cephalosporin antibiotic.
Uses
Cephalosporin antibacterial.
Definition
ChEBI: A cephalosporin prodrug having (R)-O-formylmandelamido and N-methylthiotetrazole side-groups.
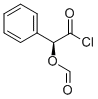
Manufacturing Process
To 21.6 kg (17.8 l) of 98% formic acid was added 1.14 kg (7.5 mols) of D-(-)-
mandelic acid and the reaction mixture was heated for 4 hours at 70°C with
stirring. The excess formic acid was evaporated off in vacuo and the residual
syrup was dissolved in 6 l of benzene. The solution was washed twice with 6 l
portions of water and was dried over magnesium sulfate. The drying agent
was filtered and washed with 1.5 l of benzene, the washes being added to the
filtrate. The dried filtrate was evaporated in vacuo to obtain the D-(-)-
mandelic acid formate ether as a syrup. The product can be crystallized from
cyclohexane to yield material melting at about 55°C to 58°C.
The mandelic acid formate ester obtained as a syrup as described above is
stirred for 2 hours with 2.9 kg (~1.75 l) of thionyl chloride at a temperature
of about 70°C. The excess thionyl chloride is removed by evaporation and the
residual green solution is vacuum distilled. The product, O-formyl mandeloyl
chloride, distills over at 127°C to 130°C (15 mm) or at 108°C to 112°C (7
mm).
To 13 l of ethyl acetate were added 85.1 g (2.59 mols) of 7-amino-3-(1-
methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-carboxylic acid and 1,361 g
(10.37 mols) of monotrimethylsilyl acetamide, and the mixture was stirred at
50°C until a clear solution was obtained. The solution was cooled to 20°C and
514 g (2.59 mold of O-formyl mandeloyl chloride was added at a rate such
that the temperature of the reaction solution was maintained between about
20°C to 25% with ice-cooling.
The reaction mixture was stirred for 1.5 hours at about room temperature
after the addition of the mandeloyl chloride was completed. Five liters of
water were then added to the reaction mixture and the diluted mixture was
stirred for about 10 minutes. The organic layer was separated and was
washed twice with water. The combined washes are extracted with 1.5 l of
ethyl acetate and the extract is combined with the washed organic layer. The
whole was dried over magnesium sulfate, filtered and evaporated in vacuo on
a 25°C water bath to yield 1,460 g of product, 7-(D-2-formyloxy-2-
phenylacetamido)-3-(1-methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-
carboxylic acid, as a yellow foam.
The product was dissolved in 5 l of acetone and the solution was mixed with a
solution of 430 g (2.59 mols) of sodium 2-ethylhexanoate in 5.4 l of acetone.
The combined solutions were seeded and stirred in an ice bath for 1.5 hours.
The crystalline precipitate of sodium 7-(D-2-formyloxy-2-phenylacetamido)-3-
(1-methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-carboxylate was filtered
and washed with 5 l of acetone. The crystalline salt was dried overnight in a
vacuum oven at 40°C to yield 1,060 g (80%)of product, melting at 182°C to
184°C.
brand name
Mandol (Lilly).
Therapeutic Function
Antibiotic
Mechanism of action
Cefamandole nafate has a formylated D-mandelic amide moiety at C-7. The formate ester is cleaved rapidly in the host to release the more active cefamandole. The esterification also apparently overcomes the instability of cefamandole when it is stored in dry form. This agent has increased activity against Haemophil us influenzae and some Gram-negative bacilli as compared with the first-generation cephalosporins. Loss of the 5-thio-l-methyl-l-H-tetrazole moiety (referred to sometimes by the acronym MT T) from C-3 is associated with prothrombin deficiency and bleeding problems as well as with an Antabuse-like acute alcohol intolerance. On the other hand, this grouping enhances potency and prevents metabolism by deacetylation. Like the other second-generation cephalosporins, cefamandole is more active against Gram-negative bacteria. The principle clinical use is for lower respiratory tract, skin and skin structures, and bone and joint infections as well as septicemia and urinary tract infections when the organisms are sensitive.
Clinical Use
The D-mandeloyl moiety of Cemandil sodium salt appears toconfer resistance to a few β-lactamases, since some β-lactamase–producing, Gram-negative bacteria (particularlyEnterobacteriaceae) that show resistance to cefazolin andother first-generation cephalosporins are sensitive tocefamandole. Additionally, it is active against some ampicillin-resistant strains of Neisseria and Haemophilus spp.Although resistance to β-lactamases may be a factor in determiningthe sensitivity of individual bacterial strains tocefamandole, an early study indicated that other factors,such as permeability and intrinsic activity, are frequentlymore important. The L-mandeloyl isomer is significantlyless active than the D-isomer.
Cemandil sodium salt Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Ganmiao New material Technology Co., LTD | +86-17332992504 +86-17332992504 | sales8@hbganmiao.com | China | 299 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Shaanxi Yikanglong Biotechnology Co., Ltd. | 17791478691 | yklbiotech@163.com | CHINA | 296 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Wuhan Chemwish Technology Co., Ltd | 027-67849912 | sales@chemwish.com | CHINA | 10821 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17365 | 58 |
View Lastest Price from Cemandil sodium salt manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-08-20 | Cemandil sodium salt
42540-40-9
|
US $10.00-7.00 / kg | 1kg | 99.9% | 20ton | Hebei Ganmiao New material Technology Co., LTD | |
 |
2024-04-29 | Cefamandole Nafate
42540-40-9
|
US $0.00 / kg | 1kg | 99% | 20tons | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2019-08-08 | Cemandil sodium salt
42540-40-9
|
US $3.00 / KG | 1KG | 99% | 100kg | Career Henan Chemical Co |
-

- Cemandil sodium salt
42540-40-9
- US $10.00-7.00 / kg
- 99.9%
- Hebei Ganmiao New material Technology Co., LTD
-

- Cefamandole Nafate
42540-40-9
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Cemandil sodium salt
42540-40-9
- US $3.00 / KG
- 99%
- Career Henan Chemical Co