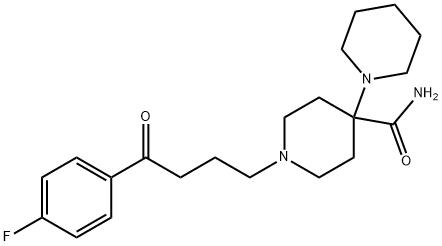
PIPAMPERONE DIHYDROCHLORIDE APPROX. 99
- CAS No.
- 1893-33-0
- Chemical Name:
- PIPAMPERONE DIHYDROCHLORIDE APPROX. 99
- Synonyms
- FPA;R 3345;D02622;Propitan;Dipiperon;Dipiperal;piperonil;Piperonyl;Dipiperone;MCN-JR-3345
- CBNumber:
- CB5497461
- Molecular Formula:
- C21H30FN3O2
- Molecular Weight:
- 375.48
- MDL Number:
- MFCD00242979
- MOL File:
- 1893-33-0.mol
- MSDS File:
- SDS
| Melting point | 126°C(lit.) |
|---|---|
| Boiling point | 563.7±50.0 °C(Predicted) |
| Density | 1.1017 (estimate) |
| storage temp. | Store at -20°C |
| solubility | H2O: ~50 mg/mL |
| pka | 16.20±0.20(Predicted) |
| form | powder |
| color | white |
| Water Solubility | 81.7mg/L at 25℃ |
| Merck | 14,7455 |
| LogP | 2.42 |
| EWG's Food Scores | 1 |
| FDA UNII | 5402501F0W |
| EPA Substance Registry System | Pipamperone (1893-33-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS09,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H411 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| RIDADR | UN 2811 6.1/PG III | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | DW1060000 | |||||||||
| HS Code | 2933.39.3100 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| NFPA 704 |
|
PIPAMPERONE DIHYDROCHLORIDE APPROX. 99 price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | P2315 | Pipamperone >98.0%(HPLC) | 1893-33-0 | 25mg | $166 | 2024-03-01 | Buy |
| TCI Chemical | P2315 | Pipamperone >98.0%(HPLC) | 1893-33-0 | 100mg | $496 | 2024-03-01 | Buy |
| Chem-Impex | 38191 | Pipamperone,98%(HPLC) 98%(HPLC) | 1893-33-0 | 25MG | $146.72 | 2021-12-16 | Buy |
| ChemScene | CS-0020023 | Pipamperone 99.89% | 1893-33-0 | 5mg | $150 | 2021-12-16 | Buy |
| AK Scientific | 0155BA | Pipamperone | 1893-33-0 | 25mg | $263 | 2021-12-16 | Buy |
PIPAMPERONE DIHYDROCHLORIDE APPROX. 99 Chemical Properties,Uses,Production
Originator
Dipiperon ,Janssen, W. Germany ,1961
Uses
Antipsychotic.
Definition
ChEBI: A member of the class of bipiperidines that is 1,4'-bipiperidine which is substituted at the 1' and 4' positions by 4-(p-fluorophenyl)-4-oxobutyl and carboxamide groups, respectively. A first generation antipsychotic, its properties are gener lly similar to those of haloperidol.
Manufacturing Process
To a stirred solution of 130.4 parts of potassium cyanide and 243.2 parts of piperidine hydrochloride in a mixture of 800 parts of water and 320 parts of ethanol is added portionwise 378 parts of 1-benzyl-4-piperidone. After about one hour a solid starts to precipitate. Stirring is continued for 24 hours. The reaction mixture is filtered and the solid is recrystallized from 1,200 parts of diisopropyl ether. On cooling to room temperature a first crop of 1-benzyl-4cyano-4-piperidinopiperidine melting at about 104°C to 106°C is obtained. By concentrating and further cooling of the mother liquor a second crop of the above compound is obtained.
A mixture of 14.1 parts of 1-benzyl-4-cyano-4-piperidinopiperidine and 40 parts of 90% sulfuric acid is heated on a steam bath for 10 minutes. Without further heating, the mixture is stirred until a temperature of about 20°C is obtained. The mixture is then poured into 150 parts of ice-water and the resultant solution is alkalized with excess ammonium hydroxide solution. The aqueous solution is decanted from the precipitated oil. On treating this oil with 80 parts of acetone, crystallization sets in. After one hour the solid is filtered off and dried to yield 1-benzyl-4-piperidinopiperidine-4-carboxamide melting at about 137.5°C to 140°C.
A mixture of 215 parts of 1-benzyl-4-piperidinopiperidine-4-carboxamide, 1,200 parts of isopropyl alcohol, 1,000 parts of distilled water and 157 parts of hydrogen chloride is debenzylated under atmospheric pressure and at a temperature of about 40°C in the presence of 40 parts of a 10% palladiumon-charcoal catalyst. After the calculated amount of hydrogen is taken up, hydrogenation is stopped. The mixture is filtered and the filtrate is evaporated. The semisolid residue is treated with a mixture of 80 parts of acetone and 80 parts of benzene and evaporated again. The residue is triturated in 200 parts of methanol and filtered, yielding the dihydrochloride of 4-piperidinopiperidine-4-carboxamide melting at about 299°C to 300.8°C with decomposition. A sample of 20 parts of the dihydrochloride is dissolved in 30 parts of water. The aqueous solution is alkalized with 15 parts of 44% sodium hydroxide and stirred for a short time. The solid obtained is filtered off yielding crude product. To separate the free base from organic and inorganic salts, it is extracted overnight in a Soxhlet apparatus with toluene. The toluene extract is evaporated and the solid residue is filtered off, yielding 4piperidinopiperidine-4-carboxamide melting at about 118.5°C to 119.5°C.
To a mixture of 4.1 parts of 4-piperidinopiperidine-4-carboxamide, 6.4 parts of sodium carbonate, and a few crystals of potassium iodide in 100 parts of anhydrous toluene is added dropwise a solution of 5.6 parts of γ-chloro-4fluorobutyrophenone and 40 parts of anhydrous toluene at a temperature of 30°C to 40°C. The mixture is stirred and refluxed for 48 hours. The reaction mixture is cooled and divided between 50 parts of water and 60 parts of chloroform. The combined organic layers - toluene and chloroform - are dried over potassium carbonate, filtered, and evaporated. The oily residue solidifies on treatment with 80 parts of ether. After cooling for 30 minutes at 0°C, there is obtained 1-[γ-(4-fluorobenzoyl)propyl]-4-piperidinopiperidine-4-carboxamide melting at about 124.5°C to 126°C.
brand name
[Name previously used: Floropipamide.].
Therapeutic Function
Antipsychotic
Safety Profile
Poison by ingestion, subcutaneous, and intravenous routes. An experimental teratogen. When heated to decomposition it emits very toxic fumes of Fand Nox.
PIPAMPERONE DIHYDROCHLORIDE APPROX. 99 Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
PIPAMPERONE DIHYDROCHLORIDE APPROX. 99 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30240 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9314 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38632 | 58 | |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49978 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
View Lastest Price from PIPAMPERONE DIHYDROCHLORIDE APPROX. 99 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Pipamperone
1893-33-0
|
US $56.00-122.00 / mg | 99.94% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Pipamperone
1893-33-0
|
US $56.00-122.00 / mg | 99.94% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-07-10 | Piperonyl butoxide
1893-33-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Pipamperone
1893-33-0
- US $56.00-122.00 / mg
- 99.94%
- TargetMol Chemicals Inc.
-

- Pipamperone
1893-33-0
- US $56.00-122.00 / mg
- 99.94%
- TargetMol Chemicals Inc.
-

- Piperonyl butoxide
1893-33-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
1893-33-0(PIPAMPERONE DIHYDROCHLORIDE APPROX. 99)Related Search:
1of4