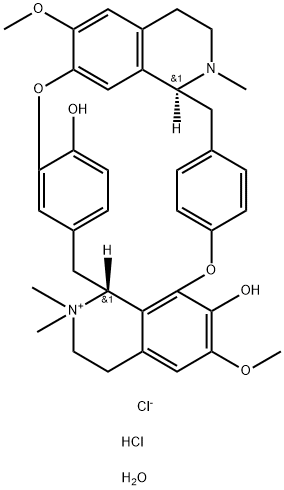
(+)-TUBOCURARINE CHLORIDE PENTAHYDRATE
- CAS No.
- 6989-98-6
- Chemical Name:
- (+)-TUBOCURARINE CHLORIDE PENTAHYDRATE
- Synonyms
- TUBOCURARINE CHLORIDE;Tubarine pentahydrate.;Tubocurarin-dichloride;TUBOCURARINE CHLORIDE 98%;Tubocurarine hydroehloride;Tubocurarine Chloride (250 mg);Tubocurarine Chloride (1702008);Tubocurarine chloride,98.0-102.0%;Tubocurarin-dichloride, Pentahydrat;TubocurarineChloridePentahydrate>
- CBNumber:
- CB5737132
- Molecular Formula:
- C37H44Cl2N2O7
- Molecular Weight:
- 699.67
- MDL Number:
- MFCD00150157
- MOL File:
- 6989-98-6.mol
- MSDS File:
- SDS
| Melting point | 275-280 °C (dec.)(lit.) |
|---|---|
| Density | 1.2074 (rough estimate) |
| refractive index | 193 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble50 mg/ml, with heating as required, clear to slightly hazy, colorless to yellow |
| form | Crystalline powder |
| color | White to Light yellow to Light orange |
| optical activity | [α]20/D +195±5°, c = 0.5% in H2O |
| Water Solubility | Soluble in water to 50mg/ml, with warming as needed. Also soluble in ethanol or methanol |
| Sensitive | Air Sensitive |
| Merck | 14,9807 |
| BRN | 3896374 |
| Stability | Hygroscopic |
| FDA UNII | 900961Z8VR |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P264-P270-P301+P310+P330-P405-P501 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25 | |||||||||
| Safety Statements | 45-36/37/39-22 | |||||||||
| RIDADR | UN 1544 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YO5100000 | |||||||||
| F | 10-23 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
(+)-TUBOCURARINE CHLORIDE PENTAHYDRATE price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 93750 | (+)-Tubocurarine chloride pentahydrate ≥97.0% (TLC) | 6989-98-6 | 1g | $427 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1702008 | Tubocurarine chloride United States Pharmacopeia (USP) Reference Standard | 6989-98-6 | 175mg | $267 | 2024-03-01 | Buy |
| TCI Chemical | C0433 | Tubocurarine Chloride Pentahydrate >98.0%(HPLC)(T) | 6989-98-6 | 1g | $304 | 2024-03-01 | Buy |
| TCI Chemical | C0433 | Tubocurarine Chloride Pentahydrate >98.0%(HPLC)(T) | 6989-98-6 | 100mg | $44 | 2021-12-16 | Buy |
| Alfa Aesar | J60222 | (+)-Tubocurarine chloride pentahydrate 98+% | 6989-98-6 | 100mg | $80.4 | 2023-06-20 | Buy |
(+)-TUBOCURARINE CHLORIDE PENTAHYDRATE Chemical Properties,Uses,Production
Description
Tubocurarine chloride is the only naturally occurring neuromuscular blocking agent. It is derived from the bark of the South American plant chondrodendron tomentosum, which has been used for centuries by South American Indians as an arrow poison. I t was the first non-depolarising neuromuscular blocking agent to be used in humans. I t has a marked propensity to produce histamine release and thus hypotension, with possibly a compensatory tachycardia. Historically, tubocurarine, alcuronium and gallamine have been used in clinical practice, but they are no longer available in the UK. A lcuronium chloride is a semisynthetic derivative of toxiferin, an alkaloid of calabash curare. Gallamine triethiodide is a trisquaternary amine. It was first used in France in 1948. The only recent use of gallamine in the UK was as a small pretreatment dose (10 mg) before suxamethonium.
Chemical Properties
white to slightly yellowish powder
Originator
Mecostrin,Squibb,US,1946
Uses
Tubocurarine is used mainly in anesthesiology as a myorelaxant, causing prolonged muscle relaxation during an operation. Small doses are successful at causing temporary relaxation of skeletal muscle without any vital change of primary body functions. It is used particularly in endotracheal intubation or orthopedic surgery for repositioning fractures, resetting compound dislocations, and so on. The main synonyms are tubarine and curarine.
Uses
muscle relaxant (skeletal)
Uses
antibacterial
Manufacturing Process
The initial step involves extraction of the stems and bark of the plant
Chondrodendron tomentosum with water as the solvent.
Producing substantially pure d-tubocurarine chloride essentially comprises
treating with picric acid the quaternary-base fraction of a crude curare of the
curarine type, hydrolyzing the resulting picrate in an emulsion of hydrochloric
acid and a water-immiscible organic solvent for picric acid, recovering
crystalline d-tubocurarine chloride from the aqueous phase, dissolving the d_x0002_tubocurarine chloride in a minimum of hot water, allowing the solution to
stand at room temperature until the bulk of the d-tubocurarine chloride
precipitates, adding sufficient concentrated hydrochloric acid to bring the HCl
content up to about 6% and refrigerating the solution.
Therapeutic Function
Muscle relaxant
General Description
Tubocurarine chloride,( )-tubocurarine chloride hydrochloride pentahydrate, isprepared from crude curare by a process of purification andcrystallization. Tubocurarine chloride occurs as a white oryellowish white to grayish white, odorless, crystalline powderthat is soluble in water. Aqueous solutions of it are stableto heat sterilization.
The structural formula for (+)-tubocurarine was longthought to be that of Ia (see structure diagram). Throughthe work of Everett et al.,the structure is now knownto be that of Ib. The monoquaternary nature of Ib thusrevealed has caused some reassessment of thinking concerningthe theoretical basis for the blocking action, becauseall had previously assumed a diquaternary structure(i.e., Ia). Nevertheless, this does not negate the earlierconclusions that a diquaternary nature of the molecule provides better blocking action than does a monoquaternarynature (e.g., Ib is approximately fourfold lesspotent than dimethyl tubocurarine iodide). Further, (+)-isotubocurarine chloride (Ic) has twice the activity of Ib inthe particular test used.
Biochem/physiol Actions
Tubocurarine is a muscle relaxant and a nicotinic acetylcholine receptor antagonist. It can induce neuromuscular blocking.
(+)-TUBOCURARINE CHLORIDE PENTAHYDRATE Preparation Products And Raw materials
(+)-TUBOCURARINE CHLORIDE PENTAHYDRATE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Alfa Chemistry | info@alfa-chemistry.com | United States | 2344 | 58 | |
| Bio Connect Life Sciences | info@bio-connect.nl | United States | 261 | 58 | |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8467 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 | Sibel@weibangbio.com | China | 6086 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 | market03@meryer.com | China | 40228 | 62 |