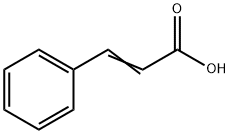
Cinnamyl alcohol
- CAS No.
- 104-54-1
- Chemical Name:
- Cinnamyl alcohol
- Synonyms
- CINNAMIC ALCOHOL;3-PHENYL-2-PROPEN-1-OL;Sterone;3-phenylprop-2-en-1-ol;TRANS-CINNAMYL ALCOHOL;(E)-3-phenyl-2-propen-1-ol;Cinnamylalkohol;3-PHENYLPROPENOL;(2E)-3-Phenyl-2-propen-1-ol;stylone
- CBNumber:
- CB5771763
- Molecular Formula:
- C9H10O
- Molecular Weight:
- 134.18
- MDL Number:
- MFCD00002921
- MOL File:
- 104-54-1.mol
- MSDS File:
- SDS
| Melting point | 30-33 °C (lit.) |
|---|---|
| Boiling point | 250 °C (lit.) |
| Density | 1.044 g/mL at 25 °C (lit.) |
| vapor density | 4.6 (vs air) |
| vapor pressure | <0.01 mm Hg ( 25 °C) |
| FEMA | 2294 | CINNAMYL ALCOHOL |
| refractive index | 1.5819 |
| Flash point | >230 °F |
| storage temp. | -20°C |
| solubility | H2O: soluble |
| form | Fused Low Melting Crystalline Solid |
| pka | 0.852[at 20 ℃] |
| Specific Gravity | 1.044 |
| color | White |
| Odor | at 100.00 %. sweet balsam hyacinth spicy green powdery cinnamic |
| Odor Type | balsamic |
| Water Solubility | 1.8 g/L (20 ºC) |
| Merck | 14,2302 |
| JECFA Number | 647 |
| BRN | 1903999 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | OOCCDEMITAIZTP-QPJJXVBHSA-N |
| LogP | 1.452 at 25℃ |
| Substances Added to Food (formerly EAFUS) | CINNAMYL ALCOHOL |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 104-54-1(CAS DataBase Reference) |
| EWG's Food Scores | 4-7 |
| FDA UNII | SS8YOP444F |
| NIST Chemistry Reference | 2-Propen-1-ol, 3-phenyl-(104-54-1) |
| EPA Substance Registry System | 3-Phenyl-2-propen-1-ol (104-54-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H317-H411 | |||||||||
| Precautionary statements | P261-P264-P273-P280-P301+P312-P302+P352 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/38-43-36 | |||||||||
| Safety Statements | 26-36/37-37/39-24-24/25 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GE2200000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29062990 | |||||||||
| Toxicity | LD50 (g/kg): 2.0 orally in rats; >5.0 dermally in rabbits (Letizia) | |||||||||
| NFPA 704 |
|
Cinnamyl alcohol price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W229407 | Cinnamyl alcohol ≥98%, FG | 104-54-1 | 1kg | $90.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | W229407 | Cinnamyl alcohol ≥98%, FG | 104-54-1 | 5kg | $290 | 2024-03-01 | Buy |
| Sigma-Aldrich | W229407 | Cinnamyl alcohol ≥98%, FG | 104-54-1 | 10Kg | $412 | 2024-03-01 | Buy |
| Sigma-Aldrich | W229407 | Cinnamyl alcohol ≥98%, FG | 104-54-1 | 25kg | $750 | 2024-03-01 | Buy |
| Sigma-Aldrich | 108197 | Cinnamyl alcohol 98% | 104-54-1 | 5g | $28.9 | 2024-03-01 | Buy |
Cinnamyl alcohol Chemical Properties,Uses,Production
Description
As an organic compound, Cinnamyl alcohol has a very distinct sweet, spicy, hyacinth odour that is found in resins, balsams and cinnamon leaves. It is used commonly in the fragrance industry due to its distinctive odour, which can be applied as a deodorant, fragrance and additive in cosmetic products and in the formulation of bath products, body and hand products, such as soaps, toothpaste, deodorants, etc. Besides, it also finds application as a food additive in chewing gum, bakery products, candy and soft drinks. Naturally, Cinnamyl alcohol is occurrent only in small amount, thus its industrial demand is usually fulfilled by chemical synthesis starting from the reduction of cinnamaldehyde.
Cinnamyl alcohol has been found to have a sensitising effect on some particular people, thus it is also considered as a Standardized Chemical Allergen. The physiologic effect of cinnamyl alcohol is caused by the Increased Histamine Release and cell-mediated Immunity.
Uses
Cinnamyl alcohol is valuable in perfumery for its odor and fixative properties. It is a component of many flower compositions (lilac, hyacinth, and lily of the valley) and is a starting material for cinnamyl esters, several of which are valuable fragrance materials. In flavor compositions, the alcohol is used for cinnamon notes and for rounding off fruit aromas.
Preparation
Cinnamyl alcohol is prepared on an industrial scale by reduction of cinnamaldehyde. Three methods are particularly useful:
1) In the Meerwein–Ponndorf reduction, cinnamaldehyde is reduced to cinnamic alcohol (yield about 85%) with isopropyl or benzyl alcohol in the presence of the corresponding aluminum alcoholate.
2) A 95% yield of Cinnamyl alcohol is obtained by selective hydrogenation of the carbonyl group in cinnamaldehydewith, for example, an osmium–carbon catalyst.
3) High yields of Cinnamyl alcohol can be obtained by reduction of cinnamaldehyde with alkali borohydrides. Formation of dihydrocinnamic alcohol is thus avoided.
References
https://en.wikipedia.org/wiki/Cinnamyl_alcohol
http://www.huidziekten.nl/allergie/stoffen/cinnamic-alcohol.htm
https://www.ulprospector.com/en/na/Food/Detail/13286/411638/Cinnamic-Alcohol
http://www.cosmeticsinfo.org/ingredient/cinnamyl-alcohol-0
https://pubchem.ncbi.nlm.nih.gov/compound/cinnamyl_alcohol#section=Top
http://www.somaiya.com/products/chemicals-pipeline/cinnamic-alcohol-1
Description
Occupational cases of contact dermatitis were reported in the perfurne industry. Patch tests can also be positive in food handlers. Cinnamic alcohol is contained in the "fragrance mix".
Chemical Properties
Cinnamyl alcohol can exist in (Z)-[4510-34-3] and (E)-[4407-36-7] forms. Although both isomers occur in nature, the (E)-isomer is far more abundant and is present, for example, in styrax oil. (E)-Cinnamyl alcohol is a colorless, crystalline solid with a hyacinth-like balsamic odor.
Cinnamyl alcohol can be dehydrogenated to give cinnamaldehyde and oxidized to give cinnamic acid. Hydrogenation yields 3-phenylpropanol and/or 3-cyclohexylpropanol. Reaction with carboxylic acids or carboxylic acid derivatives results in the formation of cinnamyl esters, some of which are used as fragrance materials.
Chemical Properties
Cinnamyl alcohol has a pleasant, floral odor and bitter taste.
Occurrence
Occurring as an ester or in the free state in hyacinth, Aristolochia clematis, Xanthorrhoea hastilis and in the essence of daffodil flowers. It is also reported found in guava fruit and peel, lemon peel oil, cassia leaf, Bourbon vanilla and cinnamon bark, leaf and root.
Uses
Cinnamic alcohol is a component in perfumed cosmetic products and deodorants; some perfumery uses (cinnamon; daffodil; hyacinth; jasmine); natural occurrence (cinnamon).
Uses
cinnamyl alcohol is naturally occurring in cinnamon bark, it can also be synthetically manufactured. It is used in cosmetics as a fragrance or flavoring agent.In perfumery; as deodorant in 12.5% solution in glycerol.
Uses
Cinnamyl alcohol was used to study the alkylation of 2,4-di-tert-butylphenol by cinnamyl alcohol using aluminum-containing mesoporous ethane-silica catalyst. It was used to study gold nanoparticles supported on titanium dioxide catalysed oxidative coupling of alcohols and amines to form the corresponding imines.
Definition
ChEBI: A primary alcohol comprising an allyl core with a hydroxy substituent at the 1-position and a phenyl substituent at the 3-position (geometry of the C2C bond unspecified).
Preparation
By reduction of cinnamic aldehyde.
Aroma threshold values
Detection: 1 ppm; cis- form, 81 ppb; trans- form, 2.8 ppm
Taste threshold values
Taste characteristics at 20 ppm: green, floral, spicy and honey with a fermented yeasty nuance.
Synthesis Reference(s)
Chemistry Letters, 5, p. 581, 1976
The Journal of Organic Chemistry, 59, p. 6378, 1994 DOI: 10.1021/jo00100a046
Tetrahedron Letters, 34, p. 257, 1993 DOI: 10.1016/S0040-4039(00)60561-0
Flammability and Explosibility
Not classified
Contact allergens
Cinnamyl alcohol occurs (in esterified form) in storax, Myroxylon pereirae, cinnamon leaves, and hyacinth oil. It is obtained by the alkaline hydrolysis of storax and prepared synthetically by reducing cinnamal diacetate with iron filings and acetic acid, and from cinnamaldehyde by Meerwein-Ponndorf reduction with aluminum isopropoxide. Cinnamyl alcohol is contained in the “fragrance mix.” As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU. Occupational cases of contact dermatitis were reported in perfume industry. Patch tests can be positive in food handlers.
Synthesis
Obtained originally by saponification of extraction from storax; synthetically, by reduction of cinnamaldehyde with sodium or potassium hydroxide.
Purification Methods
Crystallise the alcohol from diethyl ether/pentane. [Beilstein 6 I 281.]
Cinnamyl alcohol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34553 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8053 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| JINING XINHE CHEMICAL CO., LTD | +8615318402391 | sales@xinhepharma.com | China | 809 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9214 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
Related articles
- Cinnamyl Alcohol: Anti-inflammatory Effects and its Biosynthesis Method
- Cinnamyl alcohol can be successfully biosynthesized by engineered E. coli, showing potent anti-inflammatory effects by modulat....
- Jun 4,2024
View Lastest Price from Cinnamyl alcohol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Cinnamyl alcohol
104-54-1
|
US $10.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-21 | Cinnamyl alcohol
104-54-1
|
US $5.00 / KG | 1KG | ≥99% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-11-19 | Cinnamyl alcohol
104-54-1
|
US $39.00 / mL | 98.77% | 10g | TargetMol Chemicals Inc. |
-

- Cinnamyl alcohol
104-54-1
- US $10.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Cinnamyl alcohol
104-54-1
- US $5.00 / KG
- ≥99%
- Jinan Finer Chemical Co., Ltd
-

- Cinnamyl alcohol
104-54-1
- US $39.00 / mL
- 98.77%
- TargetMol Chemicals Inc.
104-54-1(Cinnamyl alcohol)Related Search:
1of4