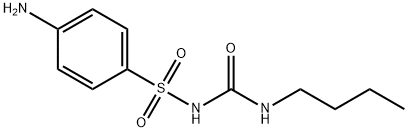
carbutamide
- CAS No.
- 339-43-5
- Chemical Name:
- carbutamide
- Synonyms
- Emedan;U-6987;Oranil;Bucrol;Burcol;Nadisan;Invenol;Alentin;Bucarban;Bukarban
- CBNumber:
- CB5883608
- Molecular Formula:
- C11H17N3O3S
- Molecular Weight:
- 271.34
- MDL Number:
- MFCD00025344
- MOL File:
- 339-43-5.mol
- MSDS File:
- SDS
| Melting point | 144-145° |
|---|---|
| Density | 1.2840 (rough estimate) |
| refractive index | 1.6630 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 5.75(H2O t=25±0.02 I=0.2) (Uncertain) |
| color | Off-White to Pale Beige |
| Water Solubility | 535.2mg/L(37 ºC) |
| FDA UNII | E3K8P4869P |
| NCI Drug Dictionary | carbutamide |
| ATC code | A10BB06 |
| EPA Substance Registry System | Benzenesulfonamide, 4-amino-N-[(butylamino)carbonyl]- (339-43-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P362-P403+P233-P501 | |||||||||
| Hazardous Substances Data | 339-43-5(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 s.c. in mice: 3 g/kg (Haack, Hagedorn) | |||||||||
| NFPA 704 |
|
carbutamide price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S385433 | N1-(BUTYLCARBAMOYL)-SULFANILAMIDE Aldrich | 339-43-5 | 50mg | $209 | 2024-03-01 | Buy |
| TRC | C183300 | Carbutamide | 339-43-5 | 5mg | $80 | 2021-12-16 | Buy |
| TRC | C183300 | Carbutamide | 339-43-5 | 25mg | $120 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FC19809 | Carbutamide | 339-43-5 | 25mg | $160 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FC19809 | Carbutamide | 339-43-5 | 10mg | $86 | 2021-12-16 | Buy |
carbutamide Chemical Properties,Uses,Production
Chemical Properties
Off-White to Pale Peach Solid
Originator
Carbutamide,Servier
Uses
Antidiabetic.
Definition
ChEBI: Carbutamide is a sulfonamide and a member of benzenes.
Manufacturing Process
223 g of the sodium salt of acetylsulfaniamide are stirred with 223 ml of
triethylene glycol. 118 g of n-butyl isothiocyanate are added to the resulting
homogeneous mixture. The resulting syrup is heated to 85°C for 4 hours. The
mixture is then stirred with 1000 ml of chloroform and 1000 ml of water. The
chloroform layer is twice shaken with water, each time with 250 ml. The
aqueous extracts are combined and rendered weakly alkaline to
phenolphthalein by addition of hydrochloric acid. Unreacted acetyl
sulfanilamide precipitates and filtered off. The filtrate is acidified to a pH of
6.5 by the addition of HCl. An oily precipitate settles from the reaction
solution and is separated therefrom. N-Butyl acetyl sulfanilylthiourea is
precipitated from mother liquors obtained thereby by addition of HCl until
Congo paper changes its color to blue. 210 g N-butyl acetyl sulfanilylthiourea
are dissolved in 1400 ml of acetone while heating. The solution is mixed with
500 ml of water. A solution of 63 g of sodium nitrite in 120 ml of water is
added thereto within about 45 minutes while stirring and cooling to 15°-20°C.
A suspension of crystals is obtained. 240 ml of 25% glacial acid are added
thereto within 30 minutes. Stirring of the mixture is continued for 6 hours. N-Butyl acetyl sulfanilylurea mixed with sulfur is precipitated and filtered off. The
crude reaction product is suspended in 1000 ml of water and is rendered
weakly alkaline to phenolphthalein. Undissolved sulfur is filtered off. The
filtrate is acidified by the addition of HCl. 250 g of N-butyl acetyl
sulfanilylthiourea having a melting point of 186°-189°C are obtained. It is
heated with 500 ml of 5 N potassium hydroxide solution to a temperature of
92°C for 2 hours while stirring. The solid reaction product is dissolved by
heating with 750 ml of water and is purified by means of activated charcoal.
The resulting solution is heated to 60°C and acidified by addition of HCl. 187 g
of N-butyl acetyl sulfanilylthiourea melting at 139°-141°C obtained thereby.
Therapeutic Function
Oral hypoglycemic
Safety Profile
A poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NO, and SOx.
carbutamide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| Nanjing Doge Biomedical Technology Co., Ltd | +86-25-58227606 +86-15305155328 | sales@dogechemical.com | China | 4128 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 | |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2123 | 70 |
View Lastest Price from carbutamide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Carbutamide
339-43-5
|
US $53.00-147.00 / mg | 98.77% | 10g | TargetMol Chemicals Inc. | ||
 |
2019-09-02 | carbutamide
339-43-5
|
US $1.00 / KG | 1KG | 98% | 1lg; 2kg; 5kg; 10kg; 100kg | Career Henan Chemical Co |
-

- Carbutamide
339-43-5
- US $53.00-147.00 / mg
- 98.77%
- TargetMol Chemicals Inc.
-

- carbutamide
339-43-5
- US $1.00 / KG
- 98%
- Career Henan Chemical Co